|
|
|
|
|
利用包含CRISPR的联合疗法治疗感染HIV的小鼠 |《自然-通讯》 |
|
|
论文标题:Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice
期刊:Nature Communications
作者:Prasanta K. Dash, Rafal Kaminski, Ramona Bella, Hang Su, Saumi Mathews, Taha M. Ahooyi, Chen Chen, Pietro Mancuso, Rahsan Sariyer, Pasquale Ferrante, Martina Donadoni, Jake A. Robinson, Brady Sillman, Zhiyi Lin, James R. Hilaire, Mary Banoub, Monalisha Elango, Nagsen Gautam, R. Lee Mosley, Larisa Y. Poluektova, JoEllyn McMillan, Aditya N. Bade, Santhi Gorantla, Ilker K. Sariyer, Tricia H. Burdo, Won-Bin Young, Shohreh Amini, Jennifer Gordon, Jeffrey M. Jacobson, Benson Edagwa, Kamel Khalili, Howard E. Gendelman
发表时间:2019/07/02
数字识别码: 10.1038/s41467-019-10366-y
原文链接:http://t.cn/AiOAUH1a
微信链接:https://mp.weixin.qq.com/s/dDrmYvs87mI6ZnbiNkPCCA
根据《自然-通讯》发表的一项研究Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice,使用一种持续的药物递送系统和基于CRISPR-Cas9的基因编辑技术进行治疗后,在一个感染HIV的小鼠子群中未检测到该病毒。总计13只小鼠在两次单独的试验中接受了联合治疗,其中5只在治疗后长达5周的时间里未出现HIV感染迹象。相比之下,在单独接受了其中某一种疗法的小鼠中,很容易就能检测到HIV。
目前,感染了HIV的病人主要依靠各种抗病毒药物进行治疗。但是,这种疗法并不能治愈病人,而且需要终身服药。
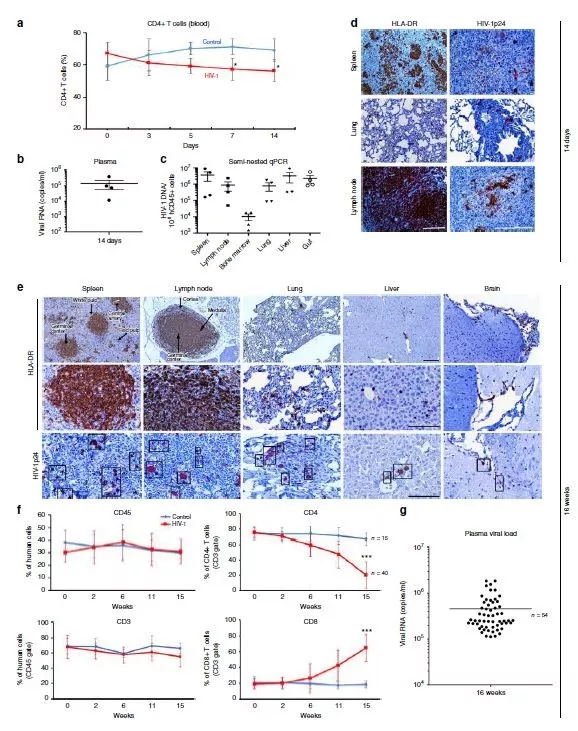
图1: 感染HIV-1的人源化小鼠体内的病毒情况及人类免疫表达情况 图源:Dash等
美国内布拉斯加大学医学中心的Howard Gendelman、美国坦普尔大学刘易斯·卡茨医学院的Kamel Khalili及同事开发了一种针对HIV的联合疗法,靶向一群受感染小鼠体内的HIV病毒。该疗法依赖于一种抗病毒药物配方和CRISPR-Cas9技术,前者会持续多天缓慢释放药物并抑制病毒活性,后者则会通过切断相关的DNA片段,消除被感染细胞中的病毒遗传密码。经过连续的治疗,近三分之一的小鼠体内没有达到可检测水平的HIV。作者使用大量不同的技术研究了小鼠,发现在接受治疗后的5周内,无法在这些小鼠的感染细胞和组织位点中检测到HIV。
虽然小鼠试验结果很有前景,但是作者计划开展进一步的研究,以改进病毒贮主体内的药物递送,并且特异性地消除潜伏的病毒感染。
摘要:Elimination of HIV-1 requires clearance and removal of integrated proviral DNA from infected cells and tissues. Here, sequential long-acting slow-effective release antiviral therapy (LASER ART) and CRISPR-Cas9 demonstrate viral clearance in latent infectious reservoirs in HIV-1 infected humanized mice. HIV-1 subgenomic DNA fragments, spanning the long terminal repeats and the Gag gene, are excised in vivo, resulting in elimination of integrated proviral DNA; virus is not detected in blood, lymphoid tissue, bone marrow and brain by nested and digital-droplet PCR as well as RNAscope tests. No CRISPR-Cas9 mediated off-target effects are detected. Adoptive transfer of human immunocytes from dual treated, virus-free animals to uninfected humanized mice fails to produce infectious progeny virus. In contrast, HIV-1 is readily detected following sole LASER ART or CRISPR-Cas9 treatment. These data provide proof-of-concept that permanent viral elimination is possible.
阅读论文全文请访问:http://t.cn/AiOAUH1a
期刊介绍:Nature Communications (https://www.nature.com/ncomms/) is an open access journal that publishes high-quality research from all areas of the natural sciences. Papers published by the journal represent important advances of significance to specialists within each field.
The 2017 journal metrics for Nature Communications are as follows:
•2-year impact factor: 12.353
•5-year impact factor: 13.691
•Immediacy index: 1.829
•Eigenfactor® score: 0.92656
•Article Influence Score: 5.684
(来源:科学网)
特别声明:本文转载仅仅是出于传播信息的需要,并不意味着代表本网站观点或证实其内容的真实性;如其他媒体、网站或个人从本网站转载使用,须保留本网站注明的“来源”,并自负版权等法律责任;作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。