|
|
|
|
|
神经发育以及去极化相关变化影响Timothy综合征中iPSC衍生神经元的转录网络 | Genome Medicine |
|
|
论文标题:Alteration in basal and depolarization induced transcriptional network in iPSC derived neurons from Timothy syndrome
期刊:Genome Medicine
作者:Yuan Tian, Irina Voineagu, Sergiu P Pa?ca, Hyejung Won, Vijayendran Chandran, Steve Horvath, Ricardo E Dolmetsch and Daniel H Geschwind
发表时间:2014/10/10
数字识别码:10.1186/s13073-014-0075-5
原文链接:
https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-014-0075-5?utm_source=WeChat&utm_medium=Social_media_organic&utm_content=DaiDen-MixedBrand-multijournal-Cell_Biology-China&utm_campaign=ORG_USG_BSCN_DD_Pluripotent_Stem_Cells_Collection
微信链接:https://mp.weixin.qq.com/s/mbjTzNT_DWjQJd_Gzyb3iw
原文作者:Yuan Tian, Irina Voineagu, Sergiu P Pa?ca, Hyejung Won, Vijayendran Chandran, Steve Horvath, Ricardo E Dolmetsch and Daniel H Geschwind
编码钙信号通路亚基的基因中常见的遗传变异和罕见突变对患多种神经精神疾病的风险具有多重影响,其中包括自闭症谱系障碍(autism spectrum disorder, ASD)和精神分裂症。为了进一步扩展已有基因的表达数据从而探寻其中的机理,来自美国加州大学洛杉矶分校的Daniel H Geschwind及其研究团队在Genome Medicine上发表了一篇名为Alteration in basal and depolarization induced transcriptional network in iPSC derived neurons from Timothy syndrome的文章,构建了一个Timothy综合征(TS)的共表达网络。
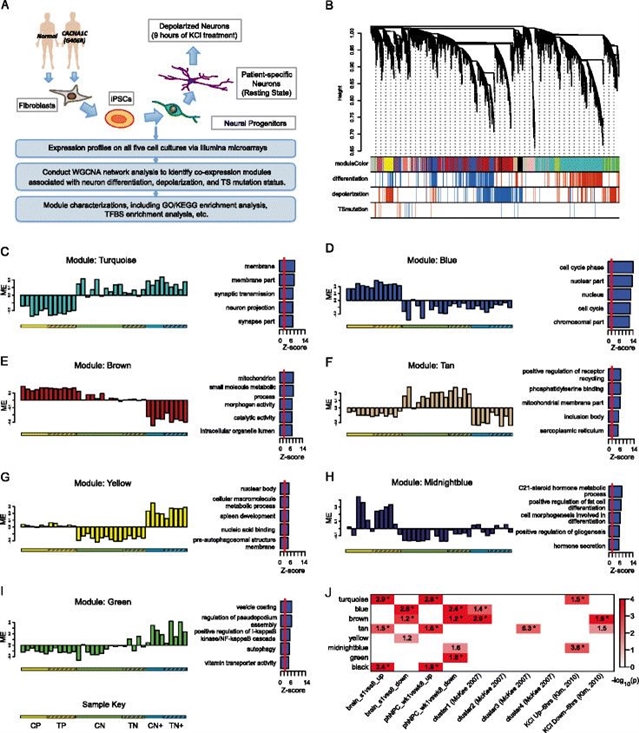
图:WGCNA鉴定了与神经发育和去极化相关的共表达模块
Yuan Tian et al.
Timothy综合征是一种具有高ASD外显率的单基因病症,通常由L型钙通道Cav1.2的突变引发。为了确定患者特异性的转录组变化,研究者们对来自于正常个体以及TS患者的(由CACNA1C的G406R变异引起)多个诱导多潜能干细胞系(induced pluripotent stem cell,iPSC)的神经祖细胞和神经元进行了全基因组的加权基因共表达网络分析(WGCNA),并采用转录因子结合位点富集分析来评估TS相关的共表达变化是否反映了钙依赖的共调节。
结果发现在TS和对照细胞系中存在保守的再生发育过程及活性相关基因的共表达模块。通过比较这两类细胞系,他们还鉴定出了能反映TS不同方面的共表达模块,包括智力残疾和ASD相关表型。此外,在整合了共表达和转录因子结合分析后,研究者们发现TS相关的转录变化可能由一些钙依赖的转录因子共同调节,这些转录因子包括NFAT,MEF2,CREB和FOXO。这一发现揭示了TS患者中钙离子信号的改变导致分子失调的相关机制。
该研究应用WGCNA首次在来自TS和对照个体的iPSC衍生神经细胞中构建了与神经发育和去极化相关的共表达网络。这些结果表明,基于基因网络的系统生物学方法有助于深入了解神经发育和作用的分子机制,并提示了钙离子信号失调的下游效应对转录的功能性影响。
摘要:
Background
Common genetic variation and rare mutations in genes encoding calcium channel subunits have pleiotropic effects on risk for multiple neuropsychiatric disorders, including autism spectrum disorder (ASD) and schizophrenia. To gain further mechanistic insights by extending previous gene expression data, we constructed co-expression networks in Timothy syndrome (TS), a monogenic condition with high penetrance for ASD, caused by mutations in the L-type calcium channel, Cav1.2.
Methods
To identify patient-specific alterations in transcriptome organization, we conducted a genome-wide weighted co-expression network analysis (WGCNA) on neural progenitors and neurons from multiple lines of induced pluripotent stem cells (iPSC) derived from normal and TS (G406R in CACNA1C) individuals. We employed transcription factor binding site enrichment analysis to assess whether TS associated co-expression changes reflect calcium-dependent co-regulation.
Results
We identified reproducible developmental and activity-dependent gene co-expression modules conserved in patient and control cell lines. By comparing cell lines from case and control subjects, we also identified co-expression modules reflecting distinct aspects of TS, including intellectual disability and ASD-related phenotypes. Moreover, by integrating co-expression with transcription factor binding analysis, we showed the TS-associated transcriptional changes were predicted to be co-regulated by calcium-dependent transcriptional regulators, including NFAT, MEF2, CREB, and FOXO, thus providing a mechanism by which altered Ca2+ signaling in TS patients leads to the observed molecular dysregulation.
Conclusions
We applied WGCNA to construct co-expression networks related to neural development and depolarization in iPSC-derived neural cells from TS and control individuals for the first time. These analyses illustrate how a systems biology approach based on gene networks can yield insights into the molecular mechanisms of neural development and function, and provide clues as to the functional impact of the downstream effects of Ca2+signaling dysregulation on transcription.
点击发现更多多功能干细胞专题论文:
https://www.biomedcentral.com/collections/stemcellreview2018?utm_source=WeChat&utm_medium=Social_media_organic&utm_content=DaiDen-MixedBrand-multijournal-Cell_Biology-China&utm_campaign=ORG_USG_BSCN_DD_Pluripotent_Stem_Cells_Collection
期刊介绍:
Genome Medicine(https://genomemedicine.biomedcentral.com/, 8.898 - 2-year Impact Factor, 8.265 - 5-year Impact Factor) is an open access journal publishing outstanding research in the application of genetics, genomics and multi-omics to understand, diagnose and treat disease. Our publication policy combines selection for broad interest and importance with a commitment to serving authors well.
(来源:科学网)
特别声明:本文转载仅仅是出于传播信息的需要,并不意味着代表本网站观点或证实其内容的真实性;如其他媒体、网站或个人从本网站转载使用,须保留本网站注明的“来源”,并自负版权等法律责任;作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。