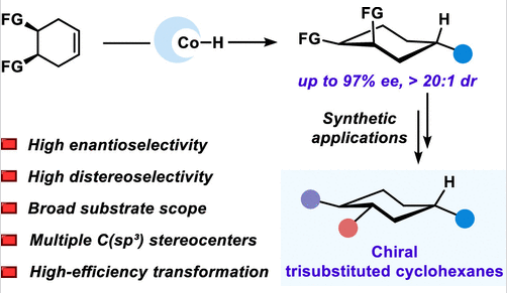
近日,西北工业大学荣子强团队报道了钴催化对映选择性脱对称氢烷基化模块化合成手性多取代环己烷。2026年1月5日,《美国化学会志》发表了这一成果。
具有高sp3特征的手性多取代六元碳环具有较强的结合亲和力、代谢稳定性和三维分子识别能力,是药物、农用化学品和天然产品中具有优势的框架。然而,这种带有多个C(sp3)立体中心的密集功能化全碳环的对映选择性构建仍然是一个主要的合成挑战。
研究组报道了一种钴催化的环己烯衍生物的不对称远程氢烷基化反应,使模块化和对映选择性地获得多取代的手性六元碳环。这种转化在温和的条件下进行,具有优异的区域和对映体选择性,允许在广泛的底物范围内进行精确的远端立体控制。所得产物具有高Fsp3含量,并且易于通过自由基C-C偶联、光氧化还原转化或Curtithem重排进行多样化。这项工作为精简合成富含sp3的立体复杂碳环提供了一种强大的策略,扩展了药物和材料发现中3D分子结构的合成工具箱。
附:英文原文
Title: Modular Synthesis of Chiral Multi-Substituted Cyclohexanes via Cobalt-Catalyzed Enantioselective Desymmetrizing Hydroalkylation
Author: Xuchao Wang, Jing Xue, Wen-Jing Yu, Siming Zheng, Mengyang Shen, Zhen Zhao, Shao-Fei Ni, Xiaohui Ji, Zhenchao Wang, Yiyun Fang, Wei Huang, Zi-Qiang Rong
Issue&Volume: January 5, 2026
Abstract: Chiral, multi-substituted six-membered carbocycles with high sp3 character are privileged frameworks in pharmaceuticals, agrochemicals, and natural products, owing to their enhanced binding affinity, metabolic stability, and three-dimensional molecular recognition. However, the enantioselective construction of such densely functionalized all-carbon rings bearing multiple C(sp3) stereocenters remains a major synthetic challenge. Here, we report a cobalt-catalyzed asymmetric remote hydroalkylation of cyclohexene derivatives, enabling modular and enantioselective access to multisubstituted, chiral six-membered carbocycles. This transformation proceeds under mild conditions with excellent regio- and enantioselectivity, allowing for precise distal stereocontrol across a broad substrate scope. The resulting products feature high Fsp3 content and are readily amenable to diversification via radical C–C coupling, photoredox transformations, or Curtius rearrangement. This work provides a powerful strategy for the streamlined synthesis of sp3-rich, stereochemically complex carbocycles, expanding the synthetic toolbox for 3D molecular architectures in drug and materials discovery.
DOI: 10.1021/jacs.5c13553
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c13553
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
