近日,复旦大学张莹团队报道了活细胞表面组赖氨酸足迹(LiFT)捕获病毒诱导的构象动力学并揭示甲型流感病毒宿主因子。该项研究成果发表在2026年1月5日出版的《美国化学会志》上。
由于受体结合的短暂性和构象性,理解细胞表面的病毒-宿主相互作用仍然是一个基本的挑战。现有的方法往往缺乏空间特异性,或者不能在蛋白质组水平上捕捉活细胞的动态结构重塑。
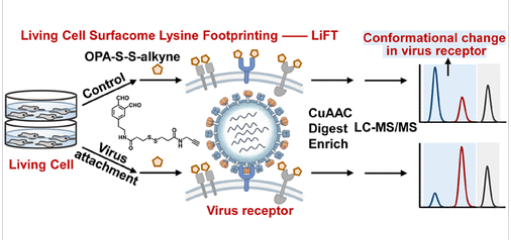
基于之前开发的细胞表面赖氨酸标记策略,研究组提出LiFT(活细胞表面赖氨酸足迹分析,一种化学蛋白质组学策略),通过分析活细胞上细胞外赖氨酸的可及性,能够直接、高特异性地绘制配体诱导的构象变化图谱。他们使用模型系统(包括HSA-布洛芬和EGFR-EGF)对LiFT进行了验证,准确检测了配体结合界面和构象动力学。
当应用于甲型流感病毒(IAV)在宿主细胞上的附着时,LiFT揭示了362种宿主细胞表面蛋白上广泛的赖氨酸可及性变化,并鉴定出胰岛素样生长因子1受体(IGF1R)为IAV的宿主因子。LiFT提供了一种与活细胞兼容且具有表面特异性的方法,用于分析功能性受体相互作用和构象动力学,为病毒入侵和受体生物学提供了新的见解。
附:英文原文
Title: Living Cell Surfacome Lysine Footprinting (LiFT) Captures Virus-Induced Conformational Dynamics and Uncovers Influenza A Virus Host Factors
Author: Shiyun Ma, Yuying Liang, Haoru Song, Ting Wang, Yuxiao Zhang, Lei Zhang, Jian Chen, Haojie Lu, Ying Zhang
Issue&Volume: January 5, 2026
Abstract: Understanding virus–host interactions at the cell surface remains a fundamental challenge due to the transient and conformational nature of receptor engagement. Existing methods often lack spatial specificity or fail to capture dynamic structural remodeling in living cells at the proteome-wide level. Building on our previously developed cell surface lysine labeling strategy, we here present LiFT (Living Cell Surfacome Lysine Footprinting), a chemical proteomic strategy enabling direct, high-specificity mapping of ligand-induced conformational changes through profiling the accessibility of extracellular lysines on living cells. LiFT was validated using model systems, including HSA–ibuprofen and EGFR-EGF, accurately detecting ligand-binding interfaces and conformational dynamics. When applied to influenza A virus (IAV) attachment on a host cell, LiFT revealed widespread lysine accessibility alterations across 362 host cell surface proteins and identified IGF1R as an IAV host factor. LiFT provides a living cell compatible and surface-specific approach for profiling functional receptor interactions and conformational dynamics, offering new insights into viral entry and receptor biology.
DOI: 10.1021/jacs.5c14196
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c14196
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
