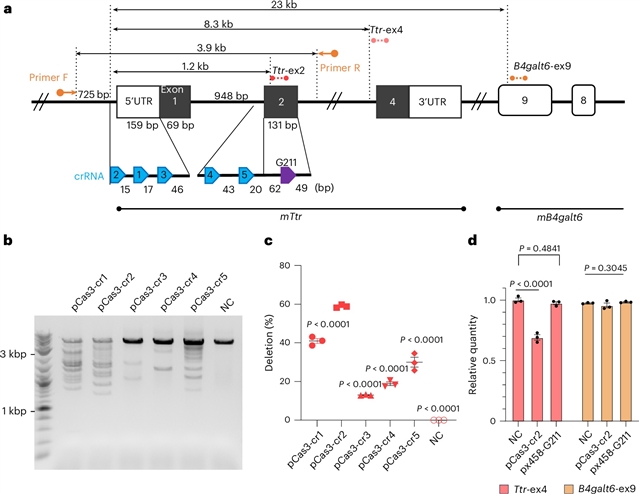
东京大学Tomoji Mashimo小组发现了基于CRISPR–Cas3编辑转甲状腺蛋白淀粉样变性小鼠模型中的靶向缺失。这一研究成果于2026年1月5日发表在国际顶尖学术期刊《自然—生物技术》上。
在这里,研究组评估了CRISPR-Cas3来纠正引导甲状腺转蛋白淀粉样变性的TTR基因突变,这是一种全身性蛋白质病,肝脏中突变TTR的丢失提供了治疗益处。通过CRISPR RNA优化,研究组在体外TTR位点实现了58.9%±0.5%的编辑,诱导了大量的缺失,从而消除了TTR的表达。Cas3产生的主要是方向性缺失,最多可达75与Cas9相反,Cas9在几个脱靶位点诱导了索引。在体内,单一的基于脂质纳米颗粒的治疗实现了48.7%±1.1%的肝脏编辑,降低了80.1%±4.6%的血清TTR水平。删除大小限制为21.1 kb。在TTR外显子人源化的小鼠中,Cas3编辑降低了无框内突变的血清TTR,并减弱了巨噬细胞相关的TTR沉积。这些发现突出表明Cas3是一种高效且独特的体内基因组编辑系统。
据悉,与Cas9相比,CRISPR-Cas3代表了一种机制上不同的基因组编辑系统,它产生远程缺失,而不是小的索引,从而降低了框内突变产生残留蛋白功能的风险。
附:英文原文
Title: CRISPR–Cas3-based editing for targeted deletions in a mouse model of transthyretin amyloidosis
Author: Ishida, Saeko, Sato, Yusuke, Chosa, Keisuke, Ezawa, Eri, Yamauchi, Yuko, Oyama, Masaaki, Kozuka-Hata, Hiroko, Ito, Rina, Sato, Rikako, Maeki, Masatoshi, Ishikawa, Tomo-o, Yamamura, Kenichi, Takeshita, Kohei, Yamaguchi, Kensuke, Kochi, Yuta, Hashiya, Fumitaka, Liu, Yiwei, Abe, Naoko, Abe, Hiroshi, Sekijima, Yoshiki, Yoshimi, Kazuto, Mashimo, Tomoji
Issue&Volume: 2026-01-05
Abstract: CRISPR–Cas3 represents a mechanistically distinct genome-editing system compared to Cas9 that generates long-range deletions rather than small indels, thereby reducing the risk of residual protein function from in-frame mutations. Here we evaluated CRISPR–Cas3 to correct mutations in the TTR gene causing transthyretin amyloidosis, a systemic proteinopathy where loss of mutant TTR in the liver offers therapeutic benefit. Through CRISPR RNA optimization we achieved 58.9%±0.5% editing at the TTR locus in vitro, inducing large deletions that abolished TTR expression. Cas3 generated mostly directional deletions up to 75kb without reproducible off-target mutations, in contrast to Cas9, which induced indels at several off-target sites. In vivo, a single lipid-nanoparticle-based treatment achieved 48.7%±1.1% hepatic editing and reduced serum TTR levels by 80.1%±4.6%. Deletion size was limited to 21kb. In TTR exon-humanized mice, Cas3 editing reduced serum TTR without in-frame mutations and attenuated macrophage-associated TTR deposition. These findings highlight Cas3 as an efficient and distinct sytem for in vivo genome editing.
DOI: 10.1038/s41587-025-02949-6
Source: https://www.nature.com/articles/s41587-025-02949-6
Nature Biotechnology:《自然—生物技术》,创刊于1996年。隶属于施普林格·自然出版集团,最新IF:68.164
官方网址:https://www.nature.com/nbt/
投稿链接:https://mts-nbt.nature.com/cgi-bin/main.plex
