近日,上海大学陈雨团队研究了用于界面程序化免疫激活和肿瘤消退的NIR驱动形态动力学2D纳米贴片。2026年1月13日,《美国化学会志》发表了这一成果。
实现免疫刺激效应的精确时空调控,仍是肿瘤免疫治疗面临的基础性瓶颈,尤其在肿瘤抗原暴露有限和免疫抑制微环境的背景下。
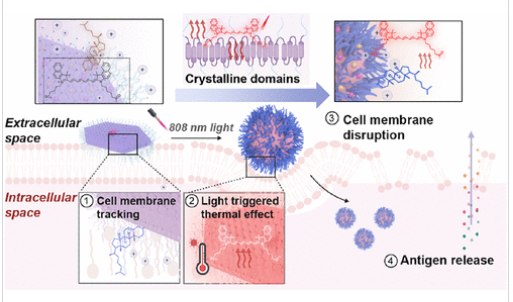
研究组提出一种光响应“动态纳米贴片”平台,通过形态引导与界面编程的免疫活化策略应对上述挑战。该纳米贴片由结晶性聚己内酯构建并整合光热转换元件,可在近红外光触发下发生从二维平面结构到零维球状结构的形态动态转变。这种动态结构转换实现了与细胞膜的可编程相互作用,构建出能原位调控癌细胞膜完整性的多功能纳米界面。在近红外照射下,纳米贴片稳定粘附于肿瘤细胞表面,引发粘附-形变-内吞的级联事件。
该过程通过诱导局部机械应力与膜扰动,促进肿瘤相关抗原和损伤相关分子模式的释放,共同启动强烈的免疫原性细胞死亡。随后抗原呈递细胞的激活可引发强效的适应性免疫应答,并扩大肿瘤微环境内的免疫细胞浸润。这种形态动态纳米贴片为癌症免疫治疗提供了高度可控的新策略,其界面编程功能范式也为精准医学、免疫治疗和生物材料工程等领域带来广泛启示。
附:英文原文
Title: NIR-Actuated Morphodynamic 2D Nanopatches for Interface-Programmed Immunoactivation and Tumor Regression
Author: Ye Wu, Wencong Jia, Tianlai Xia, Jing Liao, Wenjin He, Huijing Wang, Wei Yang, Xinyue Dai, Wei Feng, Rachel K. O’Reilly, Zaizai Tong, Meihua Yu, Yujie Xie, Yu Chen
Issue&Volume: January 13, 2026
Abstract: Achieving precise spatiotemporal modulation of immunostimulatory effects remains a fundamental barrier in tumor immunotherapy, particularly in the context of limited tumor antigen exposure and an immunosuppressive microenvironment. Herein, we present a light-responsive “dynamic nanopatch” platform that addresses these challenges through morphology-directed and interface-programmed immunoactivation. Constructed from crystalline poly(ε-caprolactone) and integrated with photothermal conversion elements, the nanopatch undergoes a near-infrared (NIR)-triggered morphology-dynamic transition from a two-dimensional planar structure to a zero-dimensional spherical counterpart. This dynamic structural transformation enables programmable interactions with the cellular membrane, establishing a versatile nanointerface capable of the in situ regulation of cancer cell membrane integrity. Upon NIR irradiation, the nanopatch stably adheres to the tumor cell surface and initiates a cascade of adhesion, deformation, and internalization events. This process promotes localized mechanical stress and membrane perturbation, enhancing the release of tumor-associated antigens and damage-associated molecular patterns, which collectively initiate potent immunogenic cell death. Subsequent activation of antigen-presenting cells leads to robust adaptive immune engagement and amplified immune cell infiltration within the tumor microenvironment. This morphodynamic nanopatch offers a highly controllable strategy for cancer immunotherapy and a new paradigm for interface-programmed functionalities with broad implications for precision medicine, immunotherapy, and biomaterial engineering.
DOI: 10.1021/jacs.5c11219
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c11219
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
