近日,东北师范大学毕锡和团队报道了热[2+2]环加成制宝石-二氟杂环[n.1.1]烷烃的途径。相关论文于2026年1月12日发表在《自然-化学》杂志上。
[2+2]环加成反应是推动合成有机化学发展的重要反应类型。然而由于基态轨道对称性的限制,传统热力学[2 + 2]环加成反应会受到对称禁阻,因此在非光化学条件下实现该反应具有挑战性。
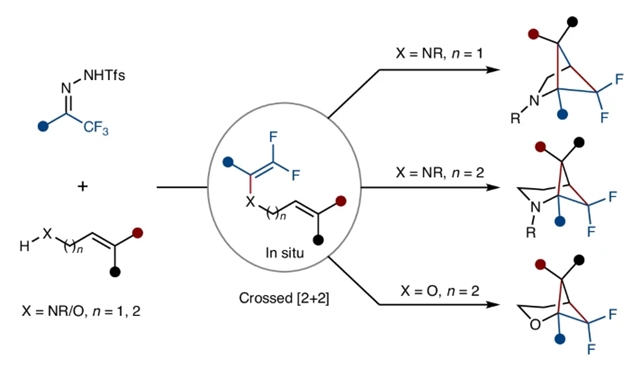
研究组报道了一种分步进行的自由基分子内热力学交叉[2 + 2]环加成反应,该反应利用原位生成的N-(丙)烯基1,1-双氟烯胺和同丙烯基1,1-双氟乙烯基醚的氟效应实现。银催化下的N-(丙)烯胺和同丙烯醇分别与三氟甲基三氟甲磺酰肼进行1,1-双氟烯基化反应后,原位生成的1,1-双氟烯烃发生分子内交叉[2 + 2]环加成反应,能够高效合成多种具有医学价值的1,1-双氟杂二环[n.1.1]烷烃,包括氮杂双环[2.1.1]己烷、氮杂双环[3.1.1]庚烷和氧杂双环[3.1.1]庚烷等结构。
该方法具有原料易得、高化学选择性/区域选择性/立体选择性、优良的功能基团兼容性和高产率等优势特征。特别值得注意的是,生成的氮杂双环[2.1.1]己烷通过氧插入可进一步转化为氮杂桥环内过氧化物,体现了这类1,1-双氟桥联氮杂双环化合物的独特性质。结合实验观测与计算研究表明,该热力学交叉[2+2]环加成反应遵循分步自由基反应机制。
附:英文原文
Title: Thermal [2+2] cycloaddition as a route to gem-difluoro heterobicyclo[n.1.1]alkanes
Author: Ning, Yongquan, Wu, Rongkai, Ning, Yongyue, Song, Qingmin, Deng, Jiahua, Jiao, Qingchi, Sivaguru, Paramasivam, Mlynarski, Jacek, de Ruiter, Graham, Hong, Xin, Bi, Xihe
Issue&Volume: 2026-01-12
Abstract: The [2+2] cycloaddition reaction has been pivotal in advancing synthetic organic chemistry. However, thermal [2+2] cycloaddition reactions are symmetry-forbidden due to ground-state orbital symmetry constraints, making them challenging to achieve under non-photochemical conditions. Here we report a stepwise radical intramolecular thermal crossed [2+2] cycloaddition, enabled by the fluorine effect of in-situ-generated N-(homo)allyl gem-difluoroenamines and homoallyl gem-difluorovinyl ethers. Silver-catalysed gem-difluoroalkenylation of N-(homo)allylamines and homoallyl alcohols with trifluoromethyl triftosylhydrazones, respectively, followed by an intramolecular crossed [2+2] cycloaddition of the in-situ-generated gem-difluoroalkenes enables the synthesis of a range of medically relevant gem-difluoro heterobicyclo[n.1.1]alkanes, including azabicyclo[2.1.1]hexanes, azabicyclo[3.1.1]heptanes and oxabicyclo[3.1.1]heptanes. This methodology features readily available starting materials, high chemo-, regio- and stereoselectivity, excellent functional group compatibility and high yields. Notably, further conversion of the formed azabicyclo[2.1.1]hexanes into azabicyclic endoperoxides via oxygen incorporation highlights the properties of these gem-difluorinated bridged azabicyclic compounds. Combined experimental and computational studies support a stepwise radical mechanism for the thermal crossed [2+2] cycloaddition.
DOI: 10.1038/s41557-025-02047-9
Source: https://www.nature.com/articles/s41557-025-02047-9
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
