近日,美国斯克里普斯研究所Ryan Shenvi团队报道了通过假定的钴硅氧烷催化丙环型硫酯的异偶联。相关论文于2026年1月12日发表在《自然-化学》杂志上。
α-氧代金属卡宾属于费歇尔卡宾家族,在多功能材料合成中具有重要应用价值。然而,传统制备方法需依赖对剧毒金属羰基配合物进行高活性有机金属试剂的加成反应。
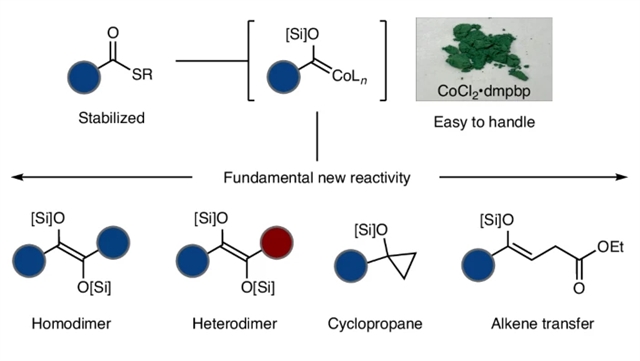
研究组报道了一种从硫酯出发经钴酰基配合物的还原性硅基化反应构建α-硅氧卡宾的新途径。该反应通过羰基二聚化过程,以高区域选择性和立体选择性合成了非对称四取代双硅氧烯烃,同时有效抑制了竞争性脱羰基副反应。所得产物可进一步转化为新型功能化片段、杂环化合物及挑战性的烯醇硅醚结构。
多种反应模式的综合分析以及机理研究的深入探讨,均表明α-氧代卡宾作为瞬态催化中间体的作用机制,证实了在温和条件下高效生成并利用此类活性物种的可行性。该方法为通过金属酰基配合物活化羧酸衍生物的卡宾化学提供了普适性平台,拓展了基于羧酸酯的功能化反应多样性。
附:英文原文
Title: Catalytic acyloin-type heterocoupling of thioesters via a putative cobalt siloxycarbene
Author: Kong, Lingran, Zong, Kevin, Guo, Jiaxu, Shenvi, Ryan
Issue&Volume: 2026-01-12
Abstract: α-Oxy-metallocarbenes exemplify Fischer carbenes and find use in the synthesis of diverse materials. Most, however, arise from the addition of reactive organometallics to toxic metal carbonyls. Here we report a method to access α-siloxycarbenes from thioesters via the reductive silylation of cobalt acyls. The reaction results in carbonyl dimerization with high hetero- and stereo-selectivity to yield unsymmetrical tetrasubstituted disiloxyalkenes, while avoiding competitive decarbonylation. These products can be further elaborated to new functionalized fragments, heterocycles and challenging enolsilanes. Several different reactivity patterns combined with mechanistic interrogation converge on α-oxycarbenes as fleeting catalytic intermediates, indicating a way to generate and exploit these reactive species under mild conditions. This method provides a general platform to harness carbene reactivity from carboxylates via metal acyls and enables a range of diverse reactivities.
DOI: 10.1038/s41557-025-02036-y
Source: https://www.nature.com/articles/s41557-025-02036-y
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
