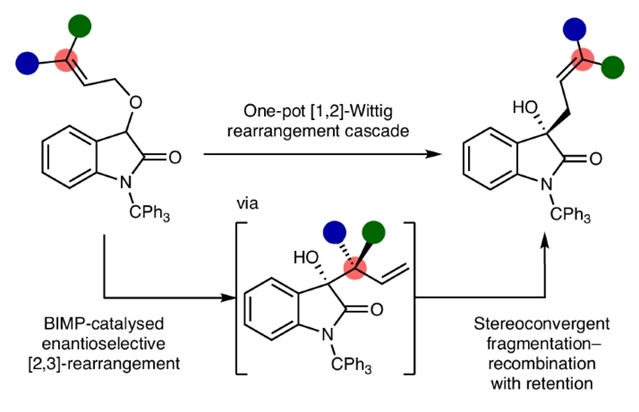
近日,英国圣安德鲁斯大学Smith, Andrew D.团队研究了烯丙醚催化对映选择性[1,2]-Wittig重排级联。该项研究成果发表在2026年1月6日出版的《自然-化学》杂志上。
烯丙醚的催化对映选择性[1,2]-Wittig重排反应是一项公认的合成挑战,因为传统上认为该反应是通过自由基对的形成和重组,经由非协同反应途径进行的。
研究组展示了应对这一挑战的催化对映选择性解决方案,证明[1,2]-Wittig产物是通过与传统教条不同的反应级联生成的。所开发的方法采用手性双功能亚氨基膦催化剂来促进初始的对映选择性[2,3]-σ迁移重排。随后,通过碱促进的、立体收敛的断裂-重组过程,该过程以高对映选择性和构型保持性进行,形式上等同于伍德沃德-霍夫曼禁阻的热[1,3]-σ迁移重排,生成[1,2]-维蒂希产物,对映体比例高达97:3。在大量量子化学计算的支持下,这一手性转移过程将对有机转化中的基础立体控制产生广泛影响。
附:英文原文
Title: The catalytic enantioselective [1,2]-Wittig rearrangement cascade of allylic ethers
Author: Kang, Tengfei, OYang, Justin, Kasten, Kevin, Allsop, Samuel S., Lewis-Atwell, Toby, Farrar, Elliot H. E., Juhl, Martin, Cordes, David B., McKay, Aidan P., Grayson, Matthew N., Smith, Andrew D.
Issue&Volume: 2026-01-06
Abstract: The catalytic enantioselective [1,2]-Wittig rearrangement of allylic ethers constitutes a recognized synthetic challenge as it is traditionally considered to arise from a non-concerted reaction pathway via formation and recombination of radical pairs. Here we show a catalytic enantioselective solution to this challenge, demonstrating that [1,2]-Wittig products are generated via an alternative reaction cascade to traditional dogma. The developed process employs a chiral bifunctional iminophosphorane catalyst to promote an initial enantioselective [2,3]-sigmatropic rearrangement. A subsequent base-promoted, stereoconvergent, fragmentation–recombination process that proceeds with high enantiospecificity and retention of configuration, formally equivalent to a Woodward–Hoffmann forbidden thermal [1,3]-sigmatropic rearrangement, generates [1,2]-Wittig products in up to 97:3 enantiomeric ratio. Supported by extensive quantum chemistry calculations, this chirality transfer process will have broad implications for fundamental stereocontrol in organic transformations.
DOI: 10.1038/s41557-025-02022-4
Source: https://www.nature.com/articles/s41557-025-02022-4
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
