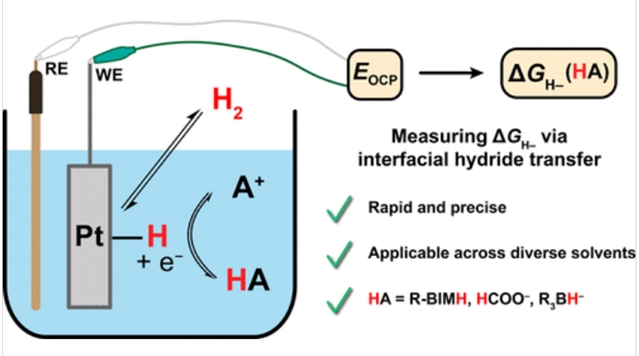
氢化物转移是整个化学价值链中必不可少的基本反应,但用于量化热力学水合性的方法有限(ΔGH-),特别是在主基团试剂中。
研究组利用易于H2活化和可逆氢化物从金属表面转移到分子试剂,即净氢还原反应(HRR),开发了一种定量主要基团试剂ΔGH-的电位法,这是传统方法难以实现的。HRR电位法首先用苯并咪唑为基础的氢化物供体进行验证,然后应用于揭示反应环境对水合度的影响。对于基于苯并咪唑的氢化物供体,HRR平衡势在不同溶剂中大致不变,表明其水合度对溶剂的依赖很大程度上反映了H-跨介质的差异溶剂化。
对于甲酸盐,HRR电位和相应的水合率在很大程度上取决于含水量。对于硼氢化物,HRR电位测定法显示,有效水合度值受氢化物受体形成的刘易斯酸碱加合物的强烈影响,但受反阳离子的影响最小。结合这些研究,阐述了HRR电位法的优点、局限性和实际考虑,强调了该方法作为测量分子水合度的补充工具的力量。
附:英文原文
Title: Reversible Interfacial Hydride Transfer as a Complementary Tool To Measure Molecular Hydricity
Author: Hye Won Chung, Hai-Xu Wang, Sai Puneet Desai, Andressa V. Müller, Sebastian Sena, Ksenija D. Glusac, Javier J. Concepcion, Yogesh Surendranath
Issue&Volume: September 29, 2025
Abstract: Hydride transfer is an essential elementary reaction across the chemical value chain, but there are limited methods available for quantifying thermodynamic hydricity (ΔGH–), particularly among main group reagents. Herein, we exploit facile H2 activation and reversible hydride transfer from a metal surface to a molecular reagent, the net hydrogen reduction reaction (HRR), to develop a potentiometric method for quantifying ΔGH– of main group reagents recalcitrant to conventional methods. HRR potentiometry is first validated with a benzimidazole-based hydride donor and then applied to uncover the impact of the reaction environment on hydricity. For a benzimidazole-based hydride donor, HRR equilibrium potentials are roughly invariant across solvents, indicating that the solvent dependence of its hydricity largely reflects the differential solvation of H– across media. For formate, HRR potentials and corresponding hydricities depend strongly on water content. For borohydrides, HRR potentiometry reveals that effective hydricity values are strongly influenced by Lewis acid–base adduct formation with the hydride acceptor but are minimally influenced by the countercation. Together with these studies, the advantages, limitations, and practical considerations of the HRR potentiometry method are discussed, highlighting the power of this methodology as a complementary tool to measure molecular hydricity.
DOI: 10.1021/jacs.5c09582
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c09582
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
