近日,东北师范大学毕锡和团队实现了催化金属卡宾插入双环[1.1.0]丁烷合成桥取代双环[1.1.1]戊烷。相关论文于2025年9月25日发表在《德国应用化学》杂志上。
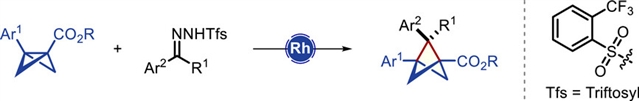
1,2,3-三取代双环[1.1.1]戊烷(BCPs)是聚取代苯的新兴饱和烃生物异构体;然而,由双环[1.1.0]丁烷(BCBs)通过直接插入金属碳来合成它们仍未实现。
研究组报道了第一个催化金属卡宾插入使用BCP的三甲酰基腙作为安全稳定的卡宾前体,这绕过了[1.1.1]推进剂在BCP支架合成中的使用。这种操作简单的方法适用于各种取代的BCB酯和三甲基腙,可以合成有价值的1,2,3-三取代的BCP,包括那些具有三氟甲基化季全碳中心的BCP,收率很高。选择性地将产物BCP酯转化为其他小环构建块应用,以及制备药物分子片段的BCP类似物,证明了该化学的多功能性和实用性。包括密度泛函理论计算在内的力学研究支持在BCB的C─C键中直接、逐步地添加金属碳。
附:英文原文
Title: Synthesis of Bridge-Substituted Bicyclo[1.1.1]Pentanes by Catalytic Metal Carbene Insertion into Bicyclo[1.1.0]Butanes
Author: Shaopeng Liu, Yongquan Ning, Wei Song, Paramasivam Sivaguru, Yixuan Wang, Ying Gan, Edward A Anderson, Xihe Bi
Issue&Volume: 2025-09-25
Abstract: 1,2,3-Trisubstituted bicyclo[1.1.1]pentanes (BCPs) are emerging saturated hydrocarbon bioisosteres for polysubstituted benzenes; however, their synthesis from bicyclo[1.1.0]butanes (BCBs) via direct metal carbene insertion remains unrealized. Herein, we report the first catalytic metal carbene insertion into BCBs using triftosylhydrazones as safe and stable carbene precursors, which circumvents the use of [1.1.1]propellane in the synthesis of the BCPs scaffold. This operationally straightforward method accommodates a wide range of substituted BCB esters and triftosylhydrazones, enabling the synthesis of valuable 1,2,3-trisubstituted BCPs, including those bearing a trifluoromethylated quaternary all-carbon center, in good-to-excellent yields. Selective post-synthetic transformations of the product BCP esters into other useful small-ring building blocks, as well as the preparation of the BCP analogue of a drug molecule fragment, demonstrate the versatility and utility of this chemistry. Mechanistic investigations, including density functional theory calculations, support a direct, stepwise metal carbene addition into the C─C bond of the BCB.
DOI: 10.1002/anie.202514232
Source: https://onlinelibrary.wiley.com/doi/10.1002/anie.202514232
Angewandte Chemie:《德国应用化学》,创刊于1887年。隶属于德国化学会,最新IF:16.823
官方网址:https://onlinelibrary.wiley.com/journal/15213773
投稿链接:https://www.editorialmanager.com/anie/default.aspx
