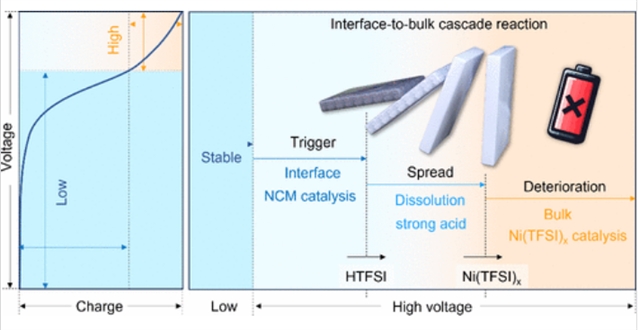
与正极材料相关的复杂寄生反应导致固体电解质严重的结构退化,阻碍了固态电池的实际应用。然而,固体电解质在阴极作用下的降解过程尚不清楚。
研究组发现电解质降解与体分解密切相关,而不像传统的观点那样只有界面催化的分解。触发事件产生的高酸性HTFSI通过体分解行为的级联反应诱导后续的Ni溶解和固体电解质体分解,导致固态电池电化学性能持续下降。通过在富镍电极上引入溶阻活性结构,减轻了体级联反应,保持了能量密度。所设计的电极在4.3 V下,在2C下循环2000次后,最大可逆容量保持了84%。这项研究为下一代长寿命电池的设计开辟了新的途径。
附:英文原文
Title: Locking Cascade Reaction Path of Bulk Degradation Achieves Stable Unmodified Solid Electrolyte
Author: Qingsong Liu, Yajie Song, Ruoyang Gao, Chuankai Fu, Hanwen An, Jiaxuan Liu, Xue Sun, Shuaifeng Lou, Geping Yin, Biao Deng, Haibo Xue, Lujun Huang, Jiajun Wang
Issue&Volume: September 17, 2025
Abstract: The severe structural degradation of solid electrolytes induced by the complex parasitic reactions associated with cathode materials has hampered the practical application of solid-state batteries. However, the degradation process of solid electrolytes influenced by the cathode is not well understood. Herein, we discover that electrolyte degradation is strongly associated with bulk decomposition, unlike conventional insight into only the interface-catalyzed decomposition. The highly acidic HTFSI produced by the triggering event can induce subsequent Ni dissolution and bulk decomposition of solid electrolytes through cascade reaction of bulk decomposition behavior, leading to a continuous decline in the electrochemical performance of solid-state batteries. By introducing a dissolution-retardant active structure onto the Ni-rich electrode, it mitigates the bulk cascade reaction and maintains the energy density. The designed electrode demonstrated an impressive 84% retention of maximum reversible capacity after 2000 cycles at 2C under 4.3 V. This study opens a new avenue in the design of next-generation long-life batteries.
DOI: 10.1021/jacs.5c04945
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c04945
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
