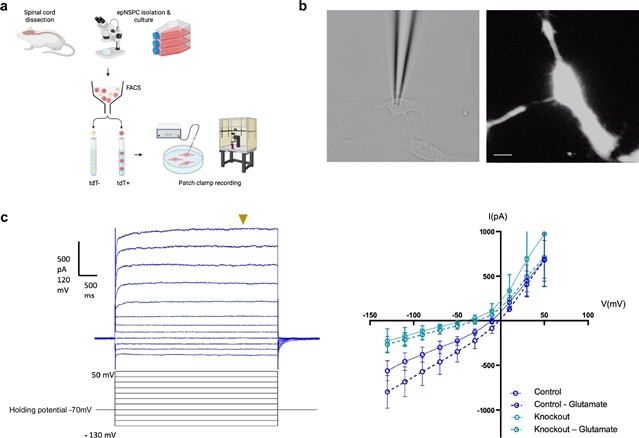
加拿大多伦多大学Michael G. Fehlings小组取得一项新突破。他们的最新研究提出了脊髓损伤后增强AMPA受体信号可增加室管膜源性神经干/祖细胞的迁移并促进功能恢复。相关论文于2025年9月15日发表在《自然—神经科学》杂志上。
在这项研究中,该研究团队确定了脊髓损伤后epNSPC调控的AMPAR依赖机制。通过对成年小鼠的谱系追踪,该课题组人员证明了在epNSPCs中有条件地敲除GluA1-GluA3 AMPAR亚基可以消除谷氨酸诱导的AMPA电流,并损害脊髓损伤后这些细胞的急性激活。用ampakine CX546增强AMPAR信号通路改变epNSPCs的转录谱,维持其在脊髓损伤后进入慢性损伤期的急性成熟逆转,增加连接蛋白43信号通路,促进其迁移能力,增强室管膜细胞与胶质细胞的接触,这可能与损伤后室管膜细胞的空间分布和迁移模式有关。CX546治疗改善了脊髓损伤后皮质脊髓束兴奋性的亚急性下降,并导致长期功能改善。总之,这项工作确定了损伤后epNSPC激活的神经递质受体依赖机制,这可能是利用脊髓再生潜力的目标。
研究人员表示,成人脊髓室管膜细胞在脊髓损伤(SCI)后被激活,获得干细胞/祖细胞特性。尽管越来越多的证据表明这些细胞在损伤后的内源性修复过程中扮演着潜在的角色,但它们激活到干细胞样状态是短暂的,不足以进行足够的再生。此外,它们激活状态的驱动因素在很大程度上仍然未知。先前的研究表明,AMPA受体(AMPARs)调节培养的室管膜源性神经干细胞/祖细胞(epNSPCs)。
附:英文原文
Title: Augmenting AMPA receptor signaling after spinal cord injury increases ependymal-derived neural stem/progenitor cell migration and promotes functional recovery
Author: Hachem, Laureen D., Moradi Chameh, Homeira, Balbinot, Gustavo, Mothe, Andrea J., Pacis, Alain, Geng Li, Rui Tong, Valiante, Taufik A., Lu, Wei, Tator, Charles H., Fehlings, Michael G.
Issue&Volume: 2025-09-15
Abstract: Ependymal cells in the adult spinal cord become activated after spinal cord injury (SCI), gaining stem/progenitor cell properties. Although growing evidence has implicated these cells as potential players in the endogenous repair process after injury, their activation to a stem-cell-like state is transient and insufficient for adequate regeneration. Moreover, the drivers of their activation state remain largely unknown. Previous work suggested that AMPA receptors (AMPARs) regulate cultured ependymal-derived neural stem/progenitor cells (epNSPCs). In this study, we identified an AMPAR-dependent mechanism of epNSPC regulation after SCI. Using lineage tracing in adult mice, we demonstrate that conditional knockout of GluA1–GluA3 AMPAR subunits in epNSPCs abolishes glutamate-induced AMPA currents and impairs the acute activation of these cells after SCI. Augmenting AMPAR signaling with the ampakine CX546 alters the transcriptional profile of epNSPCs, maintaining their acute maturation reversal after SCI into the chronic injury period, increasing connexin-43 signaling, promoting their migratory capacity and enhancing ependymal–glial cell contacts, which may contribute to the spatial distribution and migratory pattern of ependymal cells after injury. CX546 treatment ameliorates the subacute decrease in corticospinal tract excitability after SCI and leads to long-term functional improvements. Together, this work identifies a neurotransmitter receptor-dependent mechanism of epNSPC activation after injury, which may be targeted to harness the regenerative potential of the spinal cord.
DOI: 10.1038/s41593-025-02044-8
Source: https://www.nature.com/articles/s41593-025-02044-8
Nature Neuroscience:《自然—神经科学》,创刊于1998年。隶属于施普林格·自然出版集团,最新IF:28.771
官方网址:https://www.nature.com/neuro/
投稿链接:https://mts-nn.nature.com/cgi-bin/main.plex
