近日,华东师范大学杨海波团队实现了利用基于自由基的动态共价化学和超分子合成进行定向自组装。这一研究成果于2025年9月9日发表在《美国化学会志》上。
新的弱超分子相互作用和超分子合成子的发现对于指导复杂分子结构中具有更高精度、多样性和功能性的自组装过程至关重要。
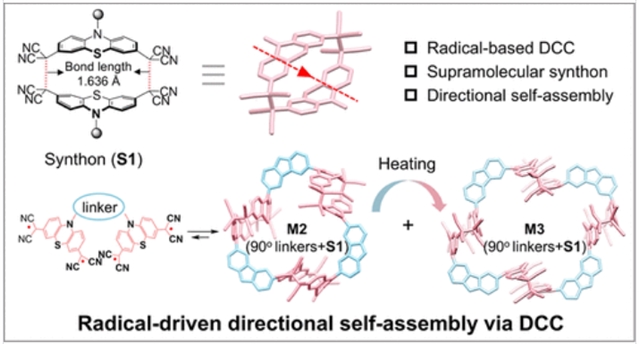
研究组报道了一种新的定向键方法,通过基于自由基的动态共价化学和超分子合成子的整合,实现了多种超分子结构的可控自组装。以两个二氰甲基取代的吩噻嗪基为基础,通过自由基为基础的动态共价键,构建了一种具有线性方向的新型大环合成物S1。通过将S1与基于菲和咔唑的不同角度的连接剂偶联,研究组实现了精确和可控的自组装。正如预期的那样,当S1与60°的菲连接体配对时,形成了一个三角形的上部结构,即大环桥接的大环M1。值得注意的是,S1与近90°咔唑连接剂偶联产生两个不同的上部结构,M2和M3,分别具有三角形和四边形几何形状。
有趣的是,合成子及其组装的上层结构都表现出有趣的热缔合和解离行为,这是由稳定的显色自由基的形成促进的。通过使用VT-UV-vis-NIR、VT-NMR和VT-EPR光谱仪对这一动态过程进行了彻底的研究。更重要的是,基于自由基的动态共价键的可逆解离和结合使M2和M3能够完全热转化。这项研究强调了将基于自由基的动态共价化学与超分子合成子相结合的潜力,作为精确构建复杂多功能超分子结构的新兴策略,为开发新型刺激响应材料铺平了道路。
附:英文原文
Title: Harnessing Radical-Based Dynamic Covalent Chemistry and Supramolecular Synthon for Directional Self-Assembly
Author: Shengzhong Li, Xiao-Li Zhao, Xueliang Shi, Hai-Bo Yang
Issue&Volume: September 9, 2025
Abstract: The discovery of new weak supramolecular interactions and supramolecular synthons is essential for directing self-assembly processes with enhanced precision, diversity, and functionality in complex molecular architectures. Here, we report the controlled self-assembly of diverse supramolecular architectures by a new directional bonding approach through the integration of radical-based dynamic covalent chemistry and supramolecular synthons. A novel macrocyclic synthon, S1, with a linear direction is constructed via radical-based dynamic covalent bonds from the phenothiazine building block substituted with two dicyanomethyl radicals. By coupling S1 with phenanthrene- and carbazole-based linkers with different angles, we achieve precise and controllable self-assembly. As anticipated, when S1 is paired with a 60° phenanthrene linker, a triangular superstructure, the macrocycle-bridged macrocycle M1, is formed. Notably, coupling S1 with a nearly 90° carbazole linker yields two distinct superstructures, M2 and M3, with triangular and quadrangular geometries, respectively. Interestingly, both the synthon and its assembled superstructures exhibit intriguing thermal association and dissociation behaviors, facilitated by the formation of stable chromogenic radicals. This dynamic process was thoroughly investigated by using VT-UV–vis–NIR, VT-NMR, and VT-EPR spectroscopies. More significantly, the reversible dissociation and association of radical-based dynamic covalent bonds enable the full thermal conversion of M2 and M3. This study highlights the potential of integrating radical-based dynamic covalent chemistry with supramolecular synthons as an emerging strategy for the precise construction of sophisticated and multifunctional supramolecular architectures, paving the way for the development of novel stimuli-responsive materials.
DOI: 10.1021/jacs.5c08506
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c08506
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
