南京大学陆红健团队近日了研究了脱胺吉斯型反应。2025年8月4日出版的《自然-化学》杂志发表了这项成果。
伯脂肪胺是许多功能分子的重要组成部分,是最容易获得的商业构建块之一。虽然它们通常被用作氮亲核试剂,但它们作为构建(sp3) C-C (sp3)键的烷基化合物的应用仍然是一个显著的挑战。
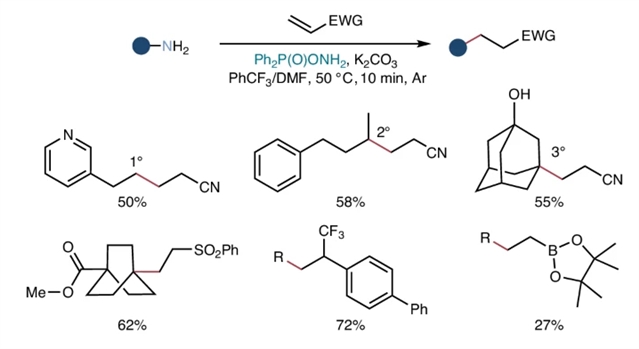
研究组提出了一种将氮原子缺失整合到aza-Michael反应中的方法,从而将经典途径从(sp3) C-N键形成重定向到(sp3) C-C (sp3)键构建。利用市售的o -二苯基磷酰羟胺作为一种高效的氮缺失试剂,该方法使多种伯脂肪胺作为烷基化合物与结构多样的缺电子烯烃偶联。
这种Giese型反应在温和条件下进行,在10分钟内完成,并表现出广泛的官能团相容性。通过桥接两个基本转换——aza-Michael反应和Giese型反应——这种方法通过统一的前体库将它们的产物空间相互连接,大大提高了合成效用。
附:英文原文
Title: Deaminative Giese-type reaction
Author: Ma, Panpan, Cui, Zhangkai, Lu, Hongjian
Issue&Volume: 2025-08-04
Abstract: Primary aliphatic amines are essential components in numerous functional molecules and rank among the most readily available commercial building blocks. Although commonly utilized as nitrogen nucleophiles, their application as alkyl sources for constructing (sp3)C–C(sp3) bonds remains a notable challenge. Here we present an approach integrating nitrogen-atom deletion into the aza-Michael reaction, thereby redirecting the classical pathway from (sp3)C–N bond formation to (sp3)C–C(sp3) bond construction. Leveraging commercially available O-diphenylphosphinylhydroxylamine as an efficient nitrogen-deletion reagent, this method enables a wide variety of primary aliphatic amines to serve as alkyl sources in couplings with structurally diverse electron-deficient olefins. This Giese-type reaction proceeds under mild conditions, achieves completion within 10min and exhibits broad functional-group compatibility. By bridging two foundational transformations—the aza-Michael reaction and the Giese-type reaction—this approach interlinks their product spaces through a unified precursor library, substantially enhancing synthetic utility.
DOI: 10.1038/s41557-025-01888-8
Source: https://www.nature.com/articles/s41557-025-01888-8
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
