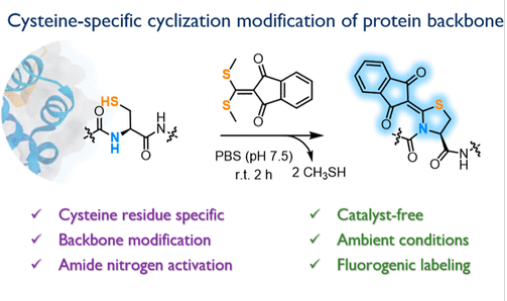
近日,西安交通大学孙晓龙团队研究了半胱氨酸残基的位点特异性化学选择性环化和荧光修饰:从侧链到主链。2025年8月28日出版的《美国化学会志》发表了这项成果。
天然蛋白质模板的选择性修饰已成为研究蛋白质结构和功能以及设计治疗性生物偶联物的有力工具。虽然在修饰蛋白质侧链和末端基团方面取得了重大进展,但由于酰胺键固有的惰性和实现位点特异性的挑战,主链修饰仍未得到充分探索。尽管主链在蛋白质功能中起着至关重要的作用,但在生理条件下对其进行选择性化学修饰已被证明是困难的。
研究组引入了一种位点特异性、化学选择性和两步策略,通过巯基/胺偶联和环化反应,通过小分子偶联受体释放挥发性甲基硫醇,对小分子半胱氨酸模拟物、肽和蛋白质进行修饰。该方法利用共轭受体的独特反应活性,首先将半胱氨酸残基偶联,然后与相邻的酰胺分子内环化,在水条件下形成五元杂环单元,无需催化剂或加热。
此外,分子动力学模拟表明,由此产生的刚性结构会引起局部主链扭转、氢键断裂和侧链取向改变,从而影响蛋白质折叠。初步研究进一步探讨了主链修饰引起的蛋白质热稳定性和酶活性的变化。更重要的是,该过程伴随着荧光开启信号,可以实时监测修饰过程。因此,这种独特的策略为骨干特异性化学修饰提供了一个新的平台,为潜在的蛋白质和肽功能研究、荧光标记和新型生物偶联物的开发铺平了道路。
附:英文原文
Title: Site-Specific Chemoselective Cyclization and Fluorogenic Modification of Protein Cysteine Residues: From Side-Chain to Backbone
Author: Hui Zhang, Ke Wei, Wanyi Yu, Youshen Wu, Xuhong Qian, Eric V. Anslyn, Xiaolong Sun
Issue&Volume: August 28, 2025
Abstract: The selective modification of natural protein templates has emerged as a powerful tool for investigating the protein structure and function as well as for designing therapeutic bioconjugates. While significant progress has been made in modifying protein side chains and terminal groups, backbone modifications remain underexplored due to the inherent inertness of amide bonds and the challenge of achieving site specificity. Despite the critical role of the backbone in the protein function, its selective chemical modification under physiological conditions has proven to be difficult. With this research, we introduce a site-specific, chemoselective, and two-step strategy for protein backbone modification via thiol/amine coupling and cyclization reactions driven by the release of volatile methyl mercaptans via a small-molecular conjugate acceptor, operating on small-molecule cysteine mimics, peptides, and proteins. This approach leverages the unique reactivity of the conjugate acceptor to first couple a cysteine residue, followed by intramolecular cyclization with an adjacent amide, forming a five-membered heterocyclic unit under aqueous conditions without requiring catalysts or heating. Additionally, molecular dynamics simulations reveal that the resulting rigid structure induces a local backbone torsion, hydrogen bond disruption, and altered side-chain orientation, thereby influencing protein folding. Preliminary investigations further explore the consequent changes in the protein thermal stability and enzymatic activity induced by backbone modification. More importantly, the process is accompanied by a fluorescence turn-on signal, enabling the real-time in situ monitoring of the modification process. Thus, this unique strategy offers a new platform for backbone-specific chemical modifications, paving the way for potential protein and peptide functional studies, fluorogenic labeling, and the development of novel bioconjugates.
DOI: 10.1021/jacs.5c08837
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c08837
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
