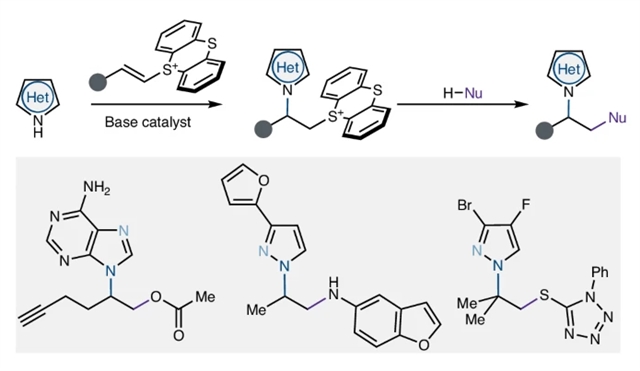
近日,美国威斯康星大学Wickens, Zachary K.团队报道了通过模块化和区域选择性N-烷基化解锁唑的化学空间。相关论文于2025年8月26日发表在《自然-化学》杂志上。
由于在从人类健康到粮食安全等领域的广泛应用,偶氮化合物是重要的合成靶标。因此,获得N官能化偶氮化合物是现代合成化学的一个重要目标。然而,令人惊讶的是,依赖于唑的N-烷基化策略从根本上限制了这些可以合成和研究的重要化合物的结构多样性。
研究组介绍了一种制备一系列重要但难以获得的N-烷基唑化合物的方法。通过引入一种碱催化氢唑化易接近的烯基噻吩亲电试剂来实现这一点。该策略通过非热可逆的C-N键形成步骤,利用了唑N-烷基化异构体之间的热力学差异,规避了唑N-烷基化区域控制的经典挑战。该反应提供了一类多功能的偶氮噻唑构建块,为研究不同的N-烷基唑分子提供了一个通用的平台。更广泛地说,通过该项目概述的独特方法有望影响各种区域选择性烷基化反应的设计和开发。
附:英文原文
Title: Unlocking azole chemical space via modular and regioselective N-alkylation
Author: Dorval, Cline, Matthews, Adrian D., Targos, Karina, Alektiar, Sara N., Holst, Dylan E., Tan, Zhifeng, Muuronen, Mikko, Diccianni, Justin B., Gmez, Jos Enrique, Sanders, Kyana M., Guzei, Ilia A., Wickens, Zachary K.
Issue&Volume: 2025-08-26
Abstract: Azoles are important synthetic targets due to their diverse applications in areas ranging from human health to food security. Accordingly, access to N-functionalized azoles is an essential goal in modern synthetic chemistry. Surprisingly, however, the relied-upon azole N-alkylation strategies fundamentally limit the structural diversity of these important compounds that can be synthesized and studied. Here we introduce an approach to prepare a broad array of important but difficult-to-access N-alkyl azole compounds. We accomplish this through the introduction of a base-catalysed hydroazolation of readily accessible alkenylthianthrenium electrophiles. This strategy circumvents the classical challenge of azole alkylation regiocontrol through an unusual reversible C–N-bond-forming step that exploits the thermodynamic differences between azole N-alkylation isomers. This reaction furnishes a class of versatile azolothianthrenium building blocks that provides a general platform to investigate diverse N-alkyl azole molecules. More broadly, the distinctive approach outlined through this project is poised to impact the design and development of diverse regioselective alkylation reactions.
DOI: 10.1038/s41557-025-01891-z
Source: https://www.nature.com/articles/s41557-025-01891-z
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
