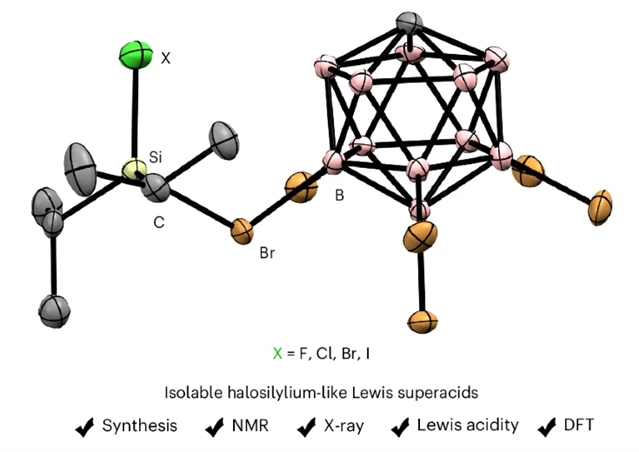
近日,德国柏林工业大学教授Oestreich, Martin团队研究了卤素取代硅离子的分离。2025年7月31日出版的《自然-化学》杂志发表了这项成果。
卤素取代的硅离子的存在和中间作用几十年来一直是人们猜测的主题。这些醚类反应中间体在合成上具有吸引力,因为它们的计算预测的超级刘易斯酸度及其在几种合成转化中的相关性,如从
研究组报道了[Alk2XSi(HCB11H5Br6)]型卤素取代硅离子(X=F、Cl、Br或I;Alk= Me, Et, iPr or tBu)的生成和表征。虽然建立的Corey氢化物转移反应不能在凝聚相中产生这些离子,但卤代硅烷Alk2XSiLG电子(LG = [H(C6H6)]+[HCB11H5Br6])提供实际接触。完整系列的反阴离子稳定iPr2XSi+阳离子被分离出来并进行了晶体学表征。得到的卤素取代的硅离子比它们已知的三烷基和氢取代的同系物具有更强的刘易斯酸,通过对它们的氟离子亲和度的定量评估来验证密度泛函理论计算。
附:英文原文
Title: Isolation of halogen-substituted silylium ions
Author: Randt, Tobias, Lehmann, Morten, Irran, Elisabeth, Kaupp, Martin, Klare, Hendrik F. T., Oestreich, Martin
Issue&Volume: 2025-07-31
Abstract: The existence and intermediacy of halogen-substituted silylium ions have been the subject of speculation for decades. These elusive reactive intermediates are synthetically attractive because of their computationally predicted super Lewis acidity and their relevance in several synthetic transformations such as recycling of waste from the Müller–Rochow process and hydrodefluorination of per- and polyfluoroalkyl substances. Here we report the generation and characterization of all halogen-substituted silylium ions of type [Alk2XSi(HCB11H5Br6)] (X=F, Cl, Br or I; Alk=Me, Et, iPr or tBu). While the established Corey hydride transfer reaction fails to make such ions in the condensed phase, the protolysis of the halosilanes Alk2XSiLG (LG=H or Ph) using Reed’s superacidic benzenium ion [H(C6H6)]+[HCB11H5Br6] provides practical access. The full series of counteranion-stabilized iPr2XSi+ cations is isolated and crystallographically characterized. The obtained halogen-substituted silylium ions are more Lewis acidic than their known trialkyl- and hydrogen-substituted congeners, as verified by quantitative assessment of their fluoride ion affinities using density functional theory calculations.
DOI: 10.1038/s41557-025-01880-2
Source: https://www.nature.com/articles/s41557-025-01880-2
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
