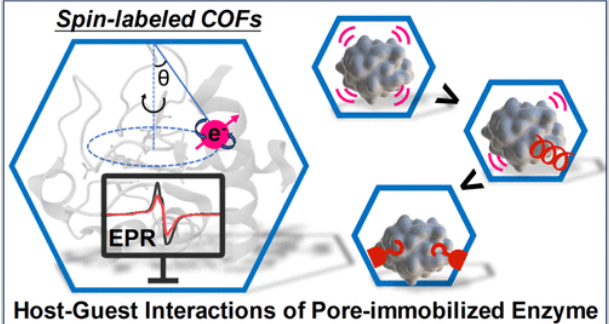
近日,中山大学周贤太团队报道了自旋传感器集成共价有机框架揭示了主-客体相互作用对固定化酶催化活性的影响。2025年7月30日出版的《美国化学会志》发表了这项成果。
在共价有机框架(COFs)内封装酶提供了一种有前景的策略,以提高酶的稳定性,促进它们的分离和回收。然而,在这种混合体系中,复杂的主客体相互作用实质上影响了酶的构象灵活性,这可以主导关键的催化过程,如底物结合和催化转化。理解这些分子间的相互作用至关重要,但仍然具有挑战性。
研究组提出了一种自旋传感器集成的COF (SSICOF)策略来探测不同酶@COF系统中的主客交互作用。这种方法利用了结构稳定的COFs的高度可编程的网状框架,其中孔隙通道通过原子工程设计,通过点击化学反应集成自旋探针。自旋探针与被封装酶之间的自旋-晶格相互作用产生电子顺磁共振(EPR)信号的可解释变化,传递传统光谱技术无法探测的主客相互作用。
利用SSICOF方法结合计算模拟,研究组证明了在COF孔约束下,固定化酶的催化活性与其自由度和结构柔韧性密切相关。该策略为探测复杂主客体系统中的潜在相互作用和预测酶@COF混合生物催化剂的催化活性提供了一个多功能和强大的工具。
附:英文原文
Title: Spin Sensor-Integrated Covalent Organic Frameworks Reveal the Effect of Host–Guest Interactions on the Catalytic Activity of Immobilized Enzymes
Author: Jingru Feng, Xiaohui Liu, Siming Huang, Hankang Zhong, Can Xue, Guosheng Chen, Xiantai Zhou, Gangfeng Ouyang
Issue&Volume: July 30, 2025
Abstract: Encapsulating enzymes within covalent organic frameworks (COFs) offers a promising strategy to enhance enzyme stability and facilitate their separation and recycling. However, the complex host–guest interactions in such hybrid systems substantially affect enzyme conformation flexibility, which can dominate key catalytic processes such as substrate binding and catalytic conversion. Understanding these molecular interplays is crucial but remains challenging. In this work, we present a spin sensor-integrated COF (SSICOF) strategy to probe host–guest interactions across diverse enzyme@COF systems. This approach leverages the highly programmable reticular framework of structurally stable COFs, wherein pore channels are atomically engineered to integrate spin probes via a click chemistry reaction. The spin–lattice interactions between the spin probes and the encapsulated enzyme generate interpretable variations in electron paramagnetic resonance (EPR) signals, delivering the host–guest interactions that cannot be probed by traditional spectroscopic techniques. Using the SSICOF approach combined with computational simulations, we demonstrate that the catalytic activity of immobilized enzymes is closely linked to their degree of freedom and structural flexibility under COF pore confinement. This strategy offers a versatile and powerful tool for probing underlying interactions in complex host–guest systems and predicting the catalytic activity of enzyme@COF hybrid biocatalysts.
DOI: 10.1021/jacs.5c06528
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c06528
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
