近日,新加坡国立大学教授孙吉及其研究团队揭示了KCNQ1门控调控KCNE1/3细胞特异性功能的机制。该研究于2025年7月31日发表于国际一流学术期刊《细胞研究》杂志上。
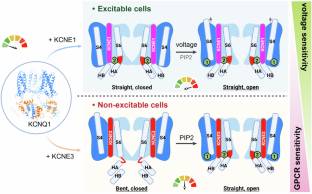
在这里,该团队分析了KCNQ1-KCNE1的结构,并重新评估了报道的KCNQ1-KCNE3的结构,无论是否有PIP2。该研究组发现KCNQ1-KCNE1/3复合物具有两个PIP2结合位点,其中KCNE1/3参与了一个以前被忽视的、未表征的位点,该位点涉及耦合电压传感器和孔域的关键残基。通过这个位点,KCNE1和KCNE3明显调节PIP2依赖性门控,以及KCNQ1的电压敏感性。因此,KCNE3将KCNQ1转化为由GPCR信号控制的电压不敏感的PIP2门控通道,以维持不可兴奋细胞中的离子稳态。KCNE1通过显著增强KCNQ1对PIP2的亲和力和对GPCR调控的抗性,与KCNQ1形成主要的电压门控通道,在可兴奋的心脏细胞中传导慢延迟整流电流。他们的研究强调了KCNE1/3如何在不同的细胞环境中调节KCNQ1门控,为组织特异性靶向多功能通道提供了见解。
据介绍,KCNQ1钾通道对心律和肠道氯离子分泌等生理过程至关重要。KCNE家族亚基(KCNE1-5)与KCNQ1结合,在各种组织中赋予不同的特性。KCNQ1的激活需要膜去极化和磷脂酰肌醇4,5-二磷酸(PIP2),其细胞水平由Gαq偶联GPCR激活控制。虽然KCNE1/3对KCNQ1电压依赖性激活的调节已经被很好地表征,但它们通过GPCR信号传导对KCNQ1 PIP2依赖性门控的影响仍然知之甚少。
附:英文原文
Title: Mechanisms of KCNQ1 gating modulation by KCNE1/3 for cell-specific function
Author: Cui, Chenxi, Zhao, Lu, Kermani, Ali A., Du, Shuzong, Pipatpolkai, Tanadet, Jiang, Meiqin, Chittori, Sagar, Tan, Yong Zi, Shi, Jingyi, Delemotte, Lucie, Cui, Jianmin, Sun, Ji
Issue&Volume: 2025-07-31
Abstract: KCNQ1 potassium channels are essential for physiological processes such as cardiac rhythm and intestinal chloride secretion. KCNE family subunits (KCNE1–5) associate with KCNQ1, conferring distinct properties across various tissues. KCNQ1 activation requires membrane depolarization and phosphatidylinositol 4,5-bisphosphate (PIP2) whose cellular levels are controlled by Gαq-coupled GPCR activation. While modulation of KCNQ1’s voltage-dependent activation by KCNE1/3 is well-characterized, their effects on PIP2-dependent gating of KCNQ1 via GPCR signaling remain less understood. Here we resolved structures of KCNQ1–KCNE1 and reassessed the reported KCNQ1–KCNE3 structures with and without PIP2. We revealed that KCNQ1–KCNE1/3 complexes feature two PIP2-binding sites, with KCNE1/3 contributing to a previously overlooked, uncharacterized site involving residues critical for coupling voltage sensor and pore domains. Via this site, KCNE1 and KCNE3 distinctly modulate the PIP2-dependent gating, in addition to the voltage sensitivity, of KCNQ1. Consequently, KCNE3 converts KCNQ1 into a voltage-insensitive PIP2-gated channel governed by GPCR signaling to maintain ion homeostasis in non-excitable cells. KCNE1, by significantly enhancing KCNQ1’s PIP2 affinity and resistance to GPCR regulation, forms predominantly voltage-gated channels with KCNQ1 for conducting the slow-delayed rectifier current in excitable cardiac cells. Our study highlights how KCNE1/3 modulates KCNQ1 gating in different cellular contexts, providing insights into tissue-specifically targeting multi-functional channels.
DOI: 10.1038/s41422-025-01152-1
Source: https://www.nature.com/articles/s41422-025-01152-1
Cell Research:《细胞研究》,创刊于1990年。隶属于施普林格·自然出版集团,最新IF:20.057
官方网址:https://www.nature.com/cr/
投稿链接:https://mts-cr.nature.com/cgi-bin/main.plex
