美国加州大学伯克利分校Alanna Schepartz团队近日研究了控制设计的微型蛋白有效内体逃逸的生物物理要求。相关论文于2025年7月8日发表在《自然-化学》杂志上。
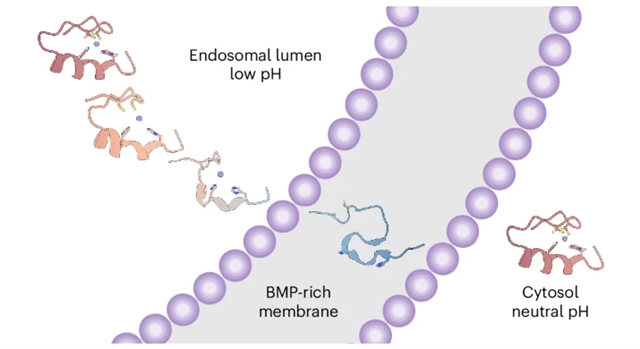
ZF5.3迷你蛋白有效地逃逸内体,引导蛋白质进入细胞质和/或细胞核。然而,除了对小型或可折叠货物和完整的HOPS复合体的要求外,人们对ZF5.3如何穿过限制性内吞膜知之甚少。研究组描述了有效内体逃逸的要求。他们确认ZF5.3在高温和pH值在5.5至7.5之间时仍保持折叠状态。在较低的pH值下,ZF5.3在Zn(II)结合His侧链质子化后协同展开,其pKa与晚期内溶酶体内腔的pKa相匹配。
pH诱导的ZF5.3的展开对于内体逃逸至关重要,因为在低pH下保持折叠的类似物无法有效地到达细胞质。一旦展开,ZF5.3就会以pH依赖的方式与双(单酰基甘油)磷酸酯相互作用,这是一种存在于晚期内溶酶体膜内小叶中的脂质。这些数据为HOPS依赖性内体逃逸提供了一个生物物理模型。
附:英文原文
Title: The biophysical requirements that govern the efficient endosomal escape of designed mini-proteins
Author: Giudice, Jonathan, Brauer, Daniel D., Zoltek, Madeline, Vzquez-Maldonado, Angel L., Dadina, Neville, Kelly, Mark, Schepartz, Alanna
Issue&Volume: 2025-07-08
Abstract: The ZF5.3 mini-protein escapes endosomes efficiently to guide proteins into the cytosol and/or nucleus. However, other than the requirement for small or unfoldable cargo and an intact HOPS complex, little is known about how ZF5.3 traverses the limiting endocytic membrane. Here we characterize the requirements for efficient endosomal escape. We confirm that ZF5.3 remains folded at high temperatures and at pH values between 5.5 and 7.5. At lower pH, ZF5.3 unfolds cooperatively upon protonation of Zn(II)-binding His side chains whose pKa matches that of the late endolysosomal lumen. pH-induced unfolding of ZF5.3 is essential for endosomal escape, as an analogue that remains folded at low pH fails to efficiently reach the cytosol. Once unfolded, ZF5.3 interacts in a pH-dependent manner with bis(monoacylglycero)phosphate, a lipid present in the inner leaflet of late endolysosomal membranes. These data provide a biophysical model for HOPS-dependent endosomal escape.
DOI: 10.1038/s41557-025-01846-4
Source: https://www.nature.com/articles/s41557-025-01846-4
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
