近日,清华大学白净卫团队发现脉冲信号和MD模拟揭示了肽段拉伸在纳米孔蛋白测序中的意义。相关论文于2025年7月3日发表在《美国化学会志》上。
目前的纳米孔蛋白测序方法缺乏对纳米孔内多肽易位动力学的详细了解,阻碍了进一步的进步和优化。
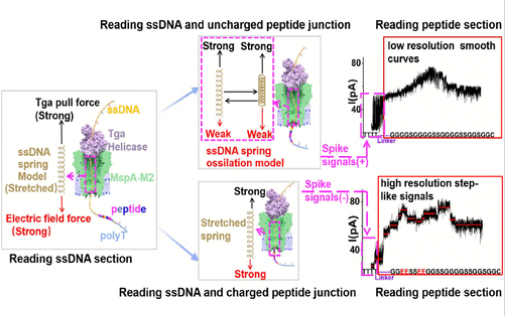
研究组发现了一组独特的尖峰信号,这些信号表征了单链DNA (ssDNA)-肽偶联物通过纳米孔的易位过程。他们提出了一个弹簧振荡模型(只表现出收缩和拉伸,而不是经典的谐波振荡或振动)来解释这些尖峰信号的产生,并通过涉及碱基损失的实验、在ssDNA中引入C6间隔层和分子动力学(MD)模拟来验证它。
此外,研究组引入带负电荷的氨基酸来诱导拉伸力稳定肽构象,停止弹簧振荡并恢复氨基酸识别的不同阶梯信号。该发现揭示了在通用肽测序中实现单氨基酸分辨率的核心难点,这可以通过在易位过程中拉伸肽来固定,从而为增强高分辨率纳米孔蛋白质测序技术铺平了道路。
附:英文原文
Title: Spike Signals and MD Simulations Reveal the Significance of Peptide Stretching in Nanopore Protein Sequencing
Author: Zhijie Chen, Jiaqi Wang, Xiaoran Meng, Fengcan Ye, Lue Wang, Yang Xu, Xinyan Wang, Qinrui Wang, Han Wen, Jingwei Bai
Issue&Volume: July 3, 2025
Abstract: Current nanopore protein sequencing methods lack detailed insights into the translocation dynamics of peptides within nanopores, impeding further advancements and optimizations. In this study, we have identified a unique set of spike signals that characterize the translocation process of a single-stranded DNA (ssDNA)-peptide conjugate through nanopores. We propose a spring oscillation model (which only exhibits contraction and stretch, not classic harmonic oscillation or vibration) to explain the generation of these spike signals and validate it through experiments involving base loss, the introduction of the C6 spacer in ssDNA, and molecular dynamics (MD) simulations. Additionally, the introduction of negatively charged amino acids to induce a stretching force stabilizes the peptide conformation, halting spring oscillations and restoring distinct step-like signals for amino acid identification. Our findings revealed the central difficulty to achieve single amino acid resolution in universal peptide sequencing, which can be fixed by stretching the peptides during translocation using additional field force, paving the way for enhancing high-resolution nanopore-based protein sequencing technologies.
DOI: 10.1021/jacs.5c00827
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c00827
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
