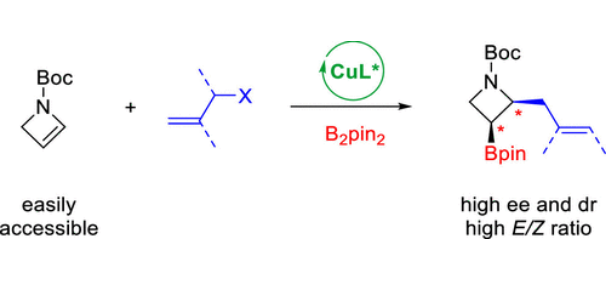
香港科技大学孙建伟团队研究了铜催化氮化硼烯丙基化合成2,3-二取代氮化氮的对映选择性。2025年6月24日,《美国化学会杂志》发表了这一成果。
研究组公开了一种高度对映选择性的氮杂环丁烷双官能化,用于方便地获得手性2,3-二取代氮杂环丁烷,这是一个以前缺乏普遍途径的重要支架系列。以铜/双膦酸盐为催化剂,在氮杂环丁烷上安装了两个多功能官能团(硼基和烯丙基),同时构建了两个新的立体中心。这代表了富电子烯烃与张力杂环中C═C键的铜催化不对称硼烷基化反应的罕见实例。
事实证明,使用烯丙基磷酸酯不仅对克服硼化烷基铜酸酯中间体对烷基化反应性低至关重要,而且对避免竞争性副反应也至关重要。值得注意的是,在几乎所有情况下,都获得了对氮杂环丁烷基序具有完全区域、对映体和非对映体选择性以及对双键构型具有良好控制的单一异构体。温和的条件显示出优异的官能团相容性和化学选择性。多功能的硼基和烯丙基官能团使产物易于转化为以前缺乏直接途径的其他有用的手性氮杂环丁烷。对照实验和动力学研究表明,反应是通过氮杂环丁烷的快速硼酰铜化反应进行的,然后通过固有控制的SN2′途径进行速率决定的烯丙基化反应。
附:英文原文
Title: Enantioselective Synthesis of 2,3-Disubstituted Azetidines via Copper-Catalyzed Boryl Allylation of Azetines
Author: Minghui Zhu, Jianwei Sun
Issue&Volume: June 24, 2025
Abstract: Disclosed here is a highly enantioselective difunctionalization of azetines for convenient access to chiral 2,3-disubstituted azetidines, a family of important scaffolds previously lacking general access. With Cu/bisphosphine as a catalyst, two versatile functionalities (boryl and allyl) were installed on azetine with concomitant construction of two new stereogenic centers. This represents a rare demonstration of Cu-catalyzed asymmetric boryl alkylation of electron-rich olefins and C═C bonds in strained heterocycles. The use of allyl phosphates proved critical not only to overcome the low reactivity of the borylated alkylcuprate intermediate toward alkylation but also to avoid competing side reactions. Remarkably, in almost all cases, single isomers were obtained with complete regio-, enantio-, and diastereoselectivies on the azetidine motif as well as excellent control on the double bond configuration. The mild conditions exhibited outstanding functional group compatibility and chemoselectivity. The versatile boryl and allyl functionalities allowed for easy transformations of the products to other useful chiral azetidines previously lacking straightforward access. Control experiments and kinetic studies indicated that the reaction proceeds by a fast boryl cupration of azetine followed by rate-determining allylation via an intrinsically controlled SN2′ pathway.
DOI: 10.1021/jacs.5c07821
Source: https://pubs.acs.org/doi/full/10.1021/jacs.5c07821
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
