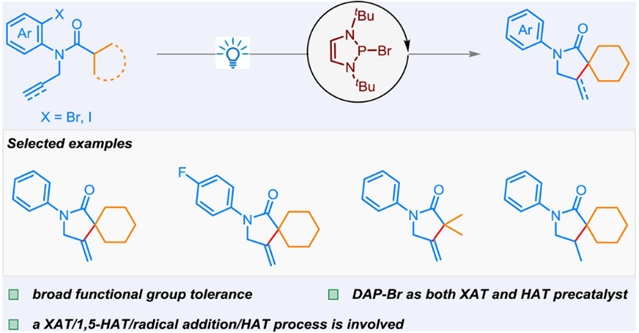
台州学院吴劼团队近日实现了重氮磷烯催化芳基卤化物1,5-HAT制备γ-螺内酰胺。这一研究成果于2025年6月9日发表在《中国化学》杂志上。
在过去的几十年里,二氮磷烯氢化物(DAP-H)已成为各种还原转化的强大催化剂。然而,以DAPs为催化剂的自由基过程极为罕见。研究组介绍了一种DAP催化的芳基卤化物自由基工艺,通过XAT/1,5-HAT/自由基加成/HAT工艺获得γ-螺内酰胺。该反应具有无金属条件和广泛的官能团耐受性,从而以良好的收率制备γ-螺内酰胺。
附:英文原文
Title: Diazaphospholene-Catalyzed 1,5-HAT of Aryl Halides to Access γ-Spirolactams
Author: Manli Zhuang, Leyi Li, Mingying Li, Yuhui Lin, Jiapian Huang, Wei Xiao, Min Yang, Jie Wu
Issue&Volume: 2025-06-09
Abstract: In the past decades, diazaphospholene hydrides (DAP-H) have emerged as powerful catalysts for a variety of reductive transformations. However, the radical processes with DAPs as catalysts are extremely rare. Herein, a DAP-catalyzed radical process of aryl halides to access γ-spirolactams through a XAT/1,5-HAT/radical addition/HAT process is presented. This reaction features metal-free conditions and broad functional group tolerance, leading to γ-spirolactams in good yields.
DOI: 10.1002/cjoc.70112
Source: https://onlinelibrary.wiley.com/doi/10.1002/cjoc.70112
Chinese Journal of Chemistry:《中国化学》,创刊于1983年。隶属于Wiley,最新IF:5.4
官方网址:https://onlinelibrary.wiley.com/journal/16147065
投稿链接:https://mc.manuscriptcentral.com/cjoc
