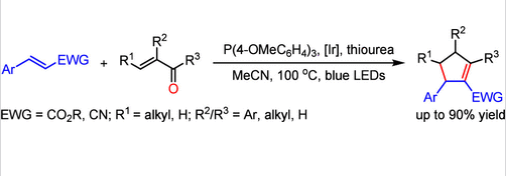
近日,浙江师范大学朱钢国团队研究了光催化膦介导的α,β-不饱和羰基和烯烃的脱氧[3 + 2]环加成。相关论文于2025年5月22日发表在《美国化学会志》上。
叔膦是一种强大的亲核试剂,能够进行许多重要的合成反应。然而,对双电子途径的完全依赖限制了磷化氢化学的发展。研究组报告了一种可见光光催化膦介导的α,β-不饱和羰基和活化烯烃的脱氧[3+2]环加成反应,该反应提供了从易于获得的起始材料中轻松获得多取代环戊烯的途径,具有良好的底物范围和有前景的产率。
机理研究证实了膦自由基阳离子的瞬时参与,以及随后由于自由基阳离子固有的亲电性而对C-C双键的α加成(α对吸电子基团),这与膦的传统亲核β加成相反。随后的单电子转移还原、迈克尔加成和1,4-质子转移级联提供了一种磷叶立德,该叶立德经历分子内维蒂希反应以完成环戊烯的构建。这项工作表明,叔膦可以作为羰基脱氧的方便试剂,并作为两种不同烯烃可控交叉偶联的无痕导向基团,为拓展膦化学和合成化学的前沿提供了新的途径。
附:英文原文
Title: Photocatalytic Phosphine-Mediated Deoxygenative [3 + 2] Cycloaddition of α,β-Unsaturated Carbonyls and Alkenes
Author: Jiayan Qiu, Xuemei Zhang, Hanliang Zheng, Gangguo Zhu
Issue&Volume: May 22, 2025
Abstract: Tertiary phosphine is a powerful nucleophile that enables numerous synthetically important reactions. However, the exclusive reliance on a two-electron pathway has restricted the development of phosphine chemistry. We report here a visible light photocatalytic phosphine-mediated deoxygenative [3 + 2] cycloaddition of α,β-unsaturated carbonyls and activated alkenes, providing facile access to polysubstituted cyclopentenes from readily accessible starting materials in promising yields with good substrate scope. Mechanistic investigations corroborate the transient involvement of a phosphine radical cation and consequent α-addition (α to electron-withdrawing groups) to C–C double bonds due to the inherent electrophilicity of the radical cation, as opposed to the traditional nucleophilic β-addition of phosphine. The ensuing single electron transfer reduction, Michael addition, and 1,4-proton transfer cascade furnish a phosphorus ylide, which undergoes an intramolecular Wittig reaction to finish the construction of the cyclopentenes. This work demonstrates that tertiary phosphine can serve as a convenient reagent for carbonyl deoxygenation and act as a traceless directing group for controllable cross-coupling of two distinct alkenes, providing new avenues to expand the frontiers of phosphine chemistry and synthetic chemistry.
DOI: 10.1021/jacs.5c03665
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c03665
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
