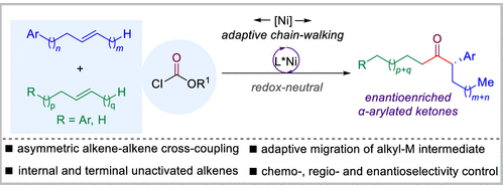
近日,南方科技大学舒伟团队实现了镍催化非活化烯烃自适应迁移的不对称交叉羰基化反应。该研究于2025年5月21日发表在《美国化学会志》上。
过渡金属催化的烷基金属物种的区域和对映选择性交叉偶联已成为现代有机合成的基石,它能够高精度地构建碳-碳和碳-杂原子键,以促进快速获得具有分子复杂性的重要有机靶标。然而,在相同条件下从一种前体选择性形成和利用不同的烷基金属中间体仍然是未知和具有挑战性的。
研究组开发了一种镍催化的非活化烯烃自适应迁移不对称交叉加氢酰化反应,用于合成富对映体的α-芳基化酮。一种烯烃通过适应性迁移作为两种不同烷基金属中间体的前体,为获得富集对映体的α-芳基化酮提供了最直接的途径之一。
附:英文原文
Title: Nickel-Catalyzed Adaptive Migration-Enabled Asymmetric Cross-Hydrocarbonylation of Unactivated Alkenes
Author: Yi-Ming Du, Jia-Ni Lin, Yu-Long Li, Qiong Yu, Wei Shu
Issue&Volume: May 21, 2025
Abstract: Transition-metal-catalyzed regio- and enantioselective cross-coupling of alkyl metallic species has emerged as a cornerstone in modern organic synthesis, which enables the construction of carbon–carbon and carbon–heteroatom bonds with high precision to facilitate rapid access to important organic targets with molecular complexity. However, the selective formation and utilization of different alkyl metallic intermediates from one precursor under identical conditions remain unknown and challenging. Herein, a Ni-catalyzed adaptive migratory asymmetric cross-hydroacylation of unactivated alkenes for the synthesis of enantioenriched α-arylated ketones has been developed. One alkene serves as a precursor for two different alkyl metallic intermediates by adaptive migration, providing one of the most straightforward pathways to access enantioenriched α-arylated ketones.
DOI: 10.1021/jacs.5c03451
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c03451
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
