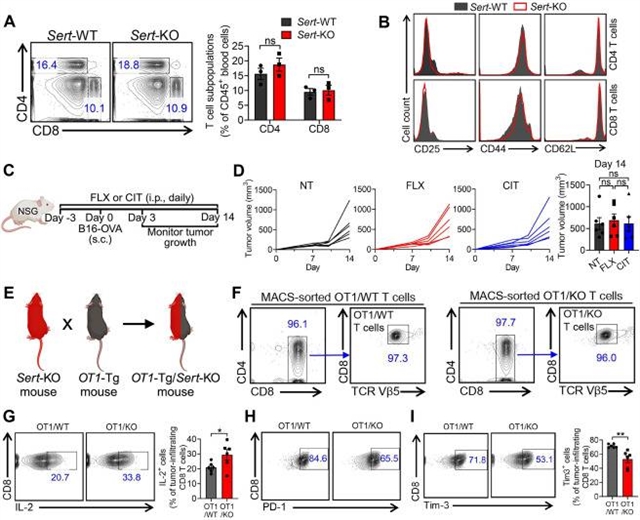
血清素转运体通过调节肿瘤内血清素轴抑制抗肿瘤免疫,这一成果由加州大学杨丽丽课题组经过不懈努力而取得。2025年5月21日出版的《细胞》发表了这项成果。
该团队报道了在肿瘤浸润的CD8 T细胞中诱导血清素转运蛋白(SERT)的作用,SERT是大脑和外周组织中血清素水平和生理功能的调节因子。SERT主题选择性5 -羟色胺再摄取抑制剂(SSRIs)是最广泛使用的抗抑郁药,在不同主题的同基因和人类异种移植肿瘤模型中,抑制SERT主题选择性5 -羟色胺再摄取抑制剂(SSRIs)显著抑制肿瘤生长并增强T细胞抗肿瘤免疫。重要的是,SSRI治疗与程序性细胞死亡蛋白1 (PD-1)阻断表现出显著的治疗协同作用,临床数据相关研究表明,在一系列癌症中,肿瘤内SERT表达与患者生存率呈负相关。从机制上讲,SERT作为一种负反馈调节剂,通过消耗肿瘤内T细胞自分泌血清素来抑制CD8 T细胞的反应性。这些发现强调了肿瘤内血清素轴的重要性,并确定了SERT作为免疫检查点,将SSRIs定位为癌症免疫治疗的有希望的候选者。
据悉,识别阻碍抗肿瘤T细胞反应的额外免疫检查点是开发下一代癌症免疫疗法的关键。
附:英文原文
Title: Serotonin transporter inhibits antitumor immunity through regulating the intratumoral serotonin axis
Author: Bo Li, James Elsten-Brown, Miao Li, Enbo Zhu, Zhe Li, Yuning Chen, Elliot Kang, Feiyang Ma, Jennifer Chiang, Yan-Ruide Li, Yichen Zhu, Jie Huang, Audrey Fung, Quentin Scarborough, Robin Cadd, Jin J. Zhou, Arnold I. Chin, Matteo Pellegrini, Lili Yang
Issue&Volume: 2025-05-21
Abstract: Identifying additional immune checkpoints hindering antitumor T cell responses is key to the development of next-generation cancer immunotherapies. Here, we report the induction of serotonin transporter (SERT), a regulator of serotonin levels and physiological functions in the brain and peripheral tissues, in tumor-infiltrating CD8 T cells. Inhibition of SERT using selective serotonin reuptake inhibitors (SSRIs), the most widely prescribed antidepressants, significantly suppressed tumor growth and enhanced T cell antitumor immunity in various mouse syngeneic and human xenograft tumor models. Importantly, SSRI treatment exhibited significant therapeutic synergy with programmed cell death protein 1 (PD-1) blockade, and clinical data correlation studies negatively associated intratumoral SERT expression with patient survival in a range of cancers. Mechanistically, SERT functions as a negative-feedback regulator inhibiting CD8 T cell reactivities by depleting intratumoral T cell-autocrine serotonin. These findings highlight the significance of the intratumoral serotonin axis and identify SERT as an immune checkpoint, positioning SSRIs as promising candidates for cancer immunotherapy.
DOI: 10.1016/j.cell.2025.04.032
Source: https://www.cell.com/cell/abstract/S0092-8674(25)00502-1
