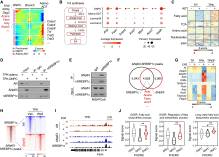
KMT2C缺陷驱动双阴性前列腺癌转分化并赋予对AR靶向治疗的抗性,这一成果由中国科学院上海营养与健康所研究员秦骏研究组经过不懈努力而取得。2025年4月24日,国际知名学术期刊《癌细胞》发表了这一成果。
该研究团队发现KMT2C突变或缺失的肿瘤在ADT后极易转变为DNPC。研究团队澄清DNPC主要源于腔细胞转分化而不是基底细胞转化。抗雄激素治疗诱导KMT2C结合AR调节基因子集的增强子,保留腺癌谱系。在AR抑制后,KMT2C通过增强子-启动子通讯维持ASPP2的表达,而其失活降低ASPP2,触发ΔNp63-dependent转分化。这种DNPC转化通过ΔNp63-mediated SREBP1c反式激活维持脂肪酸(FA)合成,通过HRAS棕榈酰化和MAPK信号激活促进DNPC生长。这些发现突出了KMT2C作为DNPC发展的表观遗传检查点,并提示靶向脂肪酸合成的治疗潜力。
据了解,双阴性前列腺癌(DNPC)以雄激素受体(AR)和神经内分泌缺失表型为特征,经常在雄激素剥夺治疗(ADT)后出现。然而,他们对DNPC的起源和调控机制的了解仍然有限。
附:英文原文
Title: KMT2C deficiency drives transdifferentiation of double-negative prostate cancer and confer resistance to AR-targeted therapy
Author: Jiacheng Guo, Ni Li, Qiuli Liu, Zongyao Hao, Guanghui Zhu, Xuege Wang, Hanling Wang, Qiang Pan, Beitao Xu, Ying Han, Guoying Zhang, Yannan Lian, Wei Zhang, Yongqiang Gu, Naiheng Lin, Xin Zeng, Zige Jin, Weihua Lan, Jun Jiang, Dong Gao, Liang Dong, Huairui Yuan, Chaozhao Liang, Jun Qin
Issue&Volume: 2025-04-24
Abstract: Double-negative prostate cancer (DNPC), characterized by an androgen receptor (AR)- and neuroendocrine-null phenotype, frequently emerges following androgen deprivation therapy (ADT). However, our understanding of the origins and regulatory mechanisms of DNPC remains limited. Here, we discover that tumors with KMT2C mutation or loss are highly susceptible to transitioning into DNPC following ADT. We clarify that DNPC primarily stems from luminal cell transdifferentiation rather than basal cell transformation. Antiandrogen treatment induces KMT2C binding at enhancers of a subset of AR-regulated genes, preserving the adenocarcinoma lineage. KMT2C maintains ASPP2 expression via enhancer-promoter communication post-AR inhibition, while its inactivation reduces ASPP2, triggering ΔNp63-dependent transdifferentiation. This DNPC transition maintains fatty acid (FA) synthesis through ΔNp63-mediated SREBP1c transactivation, fueling DNPC growth via HRAS palmitoylation and MAPK signaling activation. These findings highlight KMT2C as an epigenetic checkpoint against DNPC development and suggest the therapeutic potential of targeting fatty acid synthesis.
DOI: 10.1016/j.ccell.2025.04.002
Source: https://www.cell.com/cancer-cell/abstract/S1535-6108(25)00139-4
Cancer Cell:《癌细胞》,创刊于2002年。隶属于细胞出版社,最新IF:38.585
官方网址:https://www.cell.com/cancer-cell/home
投稿链接:https://www.editorialmanager.com/cancer-cell/default.aspx
