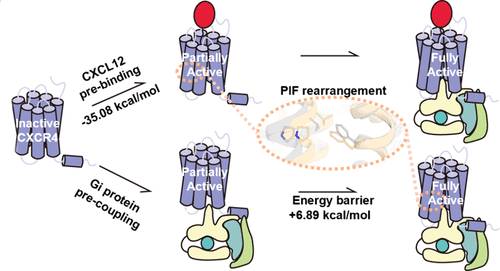
研究团队采用分子动力学模拟和他们最近开发的基于旅行推销员的自动路径搜索(TAPS)算法来有效地定位CXC趋化因子受体4 (CXCR4)与其内源性配体CXC趋化因子配体12 (CXCL12)和Gi蛋白偶联事件的最小自由能路径。小组发现,在克服了三个低能垒(3.24-6.89kcal/mol)后,即使没有CXCL12,单独的Gi也可以与CXCR4预偶联,这与先前关于载子CXCR4-Gi复合物存在的报道和他们的NanoBiT实验一致。该过程中最高能垒为6.89kcal/mol,对应于CXCR4脯氨酸-异亮氨酸-苯丙氨酸(PIF)基序的包装。有趣的是,在没有Gi的情况下,CXCL12不能单独激活CXCR4(高势垒为18.89kcal/mol)。相反,它可以通过绕过PIF填料的能量势垒来增强Gi耦合。这些结果揭示了CXCR4的激活机制,表明TAPS是揭示GPCRs完整激活途径和相应激动剂设计的有希望的工具。
研究人员表示,G蛋白偶联受体(GPCRs)的激活是一个复杂的多体多事件过程,涉及激动剂结合、受体激活、G蛋白偶联以及随后的G蛋白激活。这些事件的顺序和能量学,虽然对选择性GPCRs药物的合理设计至关重要,但很难表征,而且在很大程度上仍未得到充分探索。
附:英文原文
Title: CXC Chemokine Ligand 12 Facilitates Gi Protein Binding to CXC Chemokine Receptor 4 by Stabilizing Packing of the Proline–Isoleucine–Phenylalanine Motif: Insights from Automated Path Searching
Author: Xinyu Li, Yezhou Liu, Jinchu Liu, Wenzhuo Ma, Rujuan Ti, Arieh Warshel, Richard D. Ye, Lizhe Zhu
Issue&Volume: March 17, 2025
Abstract: The activation of G protein-coupled receptors (GPCRs) is a complex multibody multievent process involving agonist binding, receptor activation, G protein coupling, and subsequent G protein activation. The order and energetics of these events, though crucial for the rational design of selective GPCR drugs, are challenging to characterize and remain largely underexplored. Here, we employed molecular dynamics simulations and our recently developed traveling salesman-based automated path searching (TAPS) algorithm to efficiently locate the minimum free-energy paths for the coupling events of the CXC chemokine receptor 4 (CXCR4) with its endogenous ligand CXC chemokine ligand 12 (CXCL12) and Gi protein. We show that, after overcoming three low energy barriers (3.24–6.89 kcal/mol), Gi alone can precouple with CXCR4 even without CXCL12, consistent with previous reports on the existence of the apo CXCR4-Gi complex and our NanoBiT experiments. The highest barrier of 6.89 kcal/mol in this process corresponds to the packing of the proline–isoleucine–phenylalanine (PIF) motif of CXCR4. Interestingly, without Gi, CXCL12 alone cannot activate CXCR4 (high barrier of 18.89 kcal/mol). Instead, it can enhance Gi coupling by circumventing the energy barrier of PIF packing. Shedding new light on the activation mechanism of CXCR4, these results present TAPS as a promising tool for uncovering complete activation pathways of GPCRs and the corresponding agonist design.
DOI: 10.1021/jacs.4c14293
Source: https://pubs.acs.org/doi/full/10.1021/jacs.4c14293
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
