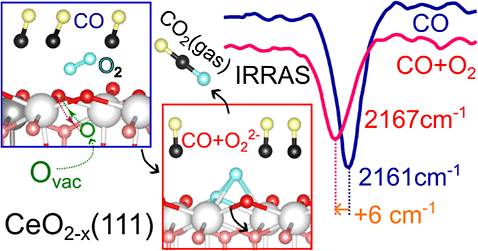
在这项研究中,该研究组介绍了红外反射-吸收光谱在块状单晶CeO2模型系统上的实验结果(111)。与预期相反,将还原表面暴露于80k的双氧(O2)中不会产生活性氧,如超氧或过氧。值得注意的是,在吸附CO存在的情况下,发生了意想不到的低温氧化反应,在氧化CeO2底物的同时消耗CO。由于在如此低的温度下,撞击的O2和吸附的CO之间不太可能发生直接反应,因此提出了一种新的机理。广泛的自旋极化密度泛函理论(DFT)计算表明,氧空位在这种低温CO氧化中起着关键作用。这些空位最初位于亚表面区域(Vss),通过与CO和O2的协同相互作用迁移到表面(Vs),导致O2活化并形成超氧或过氧物质。详细的分析确定了关键的反应中间体,并量化了它们的吸附能和激活障碍。他们的研究结果表明,与碳酸盐途径相比,过氧化物介导的途径具有较低的活化屏障,更有利于CO在低温下氧化。该研究为研究地下氧空位在气体O2和CO在CeO2上的活化机制中的动态作用提供了有价值的见解。
据了解,在金属氧化物底物上,一氧化碳(CO)和活性氧产生二氧化碳的反应机制仍然知之甚少,特别是关于氧空位的作用和活性氧吸附物的性质。
附:英文原文
Title: Synergistic Effects in Low-Temperature CO Oxidation on Cerium Oxide Surfaces
Author: Pablo G. Lustemberg, Chengwu Yang, Yuemin Wang, M. Veronica Ganduglia-Pirovano, Christof Wll
Issue&Volume: February 16, 2025
Abstract: The mechanisms underlying the reaction between carbon monoxide (CO) and activated dioxygen on metal oxide substrates to produce CO2 remain poorly understood, particularly regarding the role of oxygen vacancies and the nature of the activated O2 adsorbate. In this study, we present experimental findings from infrared reflection–absorption spectroscopy on a model system of bulk monocrystalline CeO2(111). Contrary to expectations, exposing the reduced surface to dioxygen (O2) at 80K does not yield activated oxygen species, such as superoxo or peroxo. Notably, in the presence of adsorbed CO, an unexpected low-temperature oxidation reaction occurs, consuming CO while oxidizing the CeO2 substrate. Since a direct reaction between impinging O2 and adsorbed CO is unlikely at these low temperatures, a novel mechanism is proposed. Extensive spin-polarized density functional theory (DFT) calculations reveal that oxygen vacancies play a critical role in this low-temperature CO oxidation. Initially located in the subsurface region (Vss), these vacancies migrate to the surface (Vs) via a concerted interaction with coadsorbed CO and O2, leading to O2 activation and the formation of superoxo or peroxo species. Detailed analysis identifies key reaction intermediates and quantifies their adsorption energies and activation barriers. Our findings suggest that the peroxo-mediated pathway, with its lower activation barrier, is more favorable for CO oxidation at low temperatures compared to the carbonate pathway. This study provides valuable insights into the dynamic role of subsurface oxygen vacancies in the activation of gaseous O2 and CO oxidation mechanisms on CeO2.
DOI: 10.1021/jacs.4c17658
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.4c17658
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
