近日,加拿大多伦多大学Sargent, Edward H.团队研究了CO和乙二醛共电还原促进C3产物生成。这一研究成果于2025年11月4日发表在《自然-化学》杂志上。
利用电能转化CO2与CO为高价值化学品,是一条前景广阔的可持续路径。尽管将电催化CO还原制备C1与C2产物的效率与产率已取得快速进展,但C3产物的合成仍面临重大挑战。
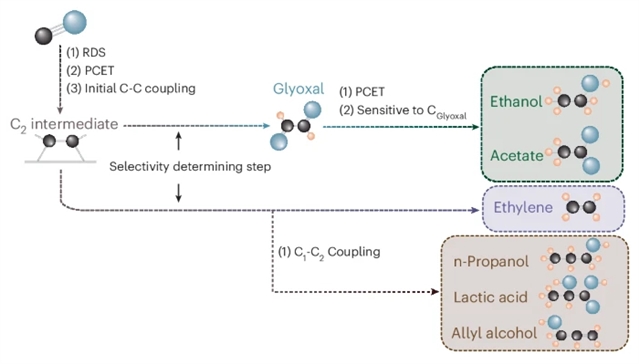
研究组通过使用探针分子与同位素标记的CO,证实C3产物的形成沿袭乙烯生成路径。研究发现,添加乙二醛可促进C3产物生成并抑制乙酸/乙醇形成,而乙二醛自身几乎不参与消耗。光谱分析表明,乙二醛的存在降低了催化剂表面CO中间体的覆盖度。
反应级数实验显示,较高的CO与OH?物种覆盖度有助于抑制乙烯路径并转向C3合成。通过协同运用两种策略——利用富OH?环境抑制乙烯生成,同时借助乙二醛阻断乙酸/乙醇路径——研究组实现了对C3产物的高选择性合成,其中法拉第效率达53%。这些发现为设计新一代C3合成电催化剂提供了重要依据。
附:英文原文
Title: Co-electroreduction of CO and glyoxal promotes C3 products
Author: Dorakhan, Roham, Sarkar, Shreya, Shirzadi, Erfan, Abbas, Hafiz Ghulam, Shayesteh, Ali, Park, Sungjin, Huang, Jianan Erick, Sinton, David, Sargent, Edward H.
Issue&Volume: 2025-11-04
Abstract: The conversion of CO2 and CO using electricity offers a promising, sustainable approach to achieve valuable products. Although CO electroreduction to C1 and C2 products has seen rapid progress in efficiency and production rate, C3 synthesis remains a major challenge. Here we show that C3 products lie along the ethylene pathway by using a probe reactant and isotope-labelled CO. We find that glyoxal addition promotes C3 formation while suppressing acetate/ethanol production, while itself scarcely being consumed. Spectroscopy reveals lower CO* coverage in the presence of glyoxal. Reaction-order experiments show higher coverages of CO* and of OH species linked to suppressing ethylene in favour of C3. By combining both strategies to suppress ethylene formation with an abundance of OH and blocking acetate/ethanol formation with glyoxal, we report a high selectivity for C3 products, including a 53% Faradaic efficiency. These insights aid the design of future catalysts for C3 production.
DOI: 10.1038/s41557-025-01985-8
Source: https://www.nature.com/articles/s41557-025-01985-8
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
