近日,中国科学院过程工程研究所袁成前团队研究了具有无酶代谢和前生物稳健性的熵驱动氨基酸凝聚体。相关论文发表在2025年11月24日出版的《美国化学会志》上。
原细胞若具备非酶催化代谢和环境适应能力,将是理解细胞生命起源的关键模型。然而,现有原细胞设计往往缺乏解释力,难以说明在早期地球条件下功能性超分子组装体如何形成,既缺乏稳健性,又缺乏前生物相关性。
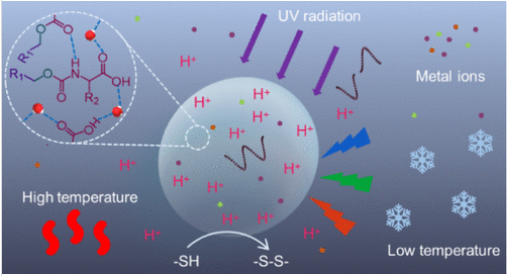
研究组证明,一些简单的氨基酸衍生物(已在地外天体和模拟前生物地球条件下观测到)可通过熵驱动的液-液相分离,经自聚集作用形成无膜原细胞。这些凝聚体微滴通过协同效应增强酶-free反应:既选择性富集代谢物,又在界面处加速反应,包括硫代谢和前生物色素合成。这些原细胞由水介导的氢键网络稳定,展现出对前生物合理压力源的卓越耐受性——如高盐度(最高4.0 M NaCl)、高浓度二价阳离子(4.0 M Mg2+/Ca2+)、紫外线辐射和极端温度波动——这些因素通常会破坏现有囊泡系统。
值得注意的是,这些结构能在界面自主产生并维持质子梯度(ΔpH ≈ 0.6–2.1),通过Na+–H+反向转运实现原始化学渗透偶联。它们还会对突发环境变化自适应重塑为致密球形形态,从而保持结构完整性。通过将区室化、非酶催化、能量转换和抗压能力整合于极简的氨基酸框架中,研究组确立了原细胞形成和存续的地质化学合理路径。这项工作凸显了凝聚体微区室在前生物条件下维持生物化学复杂性,从而架起非生命与生命系统之间桥梁的潜力。
附:英文原文
Title: Entropy-Driven Amino Acid-Based Coacervates with Enzyme-Free Metabolism and Prebiotic Robustness
Author: Shuai Cao, Guangle Li, Peng Zhou, Ehud Gazit, Xuehai Yan, Chengqian Yuan
Issue&Volume: November 24, 2025
Abstract: Protocells capable of nonenzymatic metabolism and environmental adaptation are essential models for understanding the emergence of cellular life. However, existing protocell designs often lack the robustness or prebiotic relevance to explain how functional supramolecular assemblies could have formed under early Earth conditions. In this study, we demonstrate that simple amino acid derivatives, observed on extraterrestrial bodies and under simulated prebiotic Earth conditions, undergo entropy-driven liquid–liquid phase separation to form membraneless protocells through a self-coacervation process. The synergistic effect of selective enrichment of metabolites and interfacial acceleration in these coacervate microdroplets enhances enzyme-free reactions, including sulfur metabolism and prebiotic pigment synthesis. The protocells are stabilized by water-mediated hydrogen-bonding networks and exhibit exceptional resilience to prebiotically plausible stressors─such as high salinity (up to 4.0 M NaCl), high concentrations of divalent cations (4.0 M Mg2+/Ca2+), UV radiation, and extreme temperature fluctuations─which typically disrupt existing vesicle-based systems. Remarkably, these structures autonomously generate and maintain a proton gradient (ΔpH ≈ 0.6–2.1) across their interfaces, enabling primitive chemiosmotic coupling via Na+–H+ antiport activity. They also adaptively remodel into compact spherical morphologies in response to sudden environmental changes, thereby preserving structural integrity. By integrating compartmentalization, nonenzymatic catalysis, energy transduction, and stress tolerance within a minimalist amino acid framework, our results establish a geochemically plausible pathway for the formation and persistence of functional protocells. This work highlights the potential of coacervate-based microcompartments to bridge nonliving and living systems by sustaining biochemical complexity under prebiotic conditions.
DOI: 10.1021/jacs.5c15328
Source: https://pubs.acs.org/doi/full/10.1021/jacs.5c15328
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
