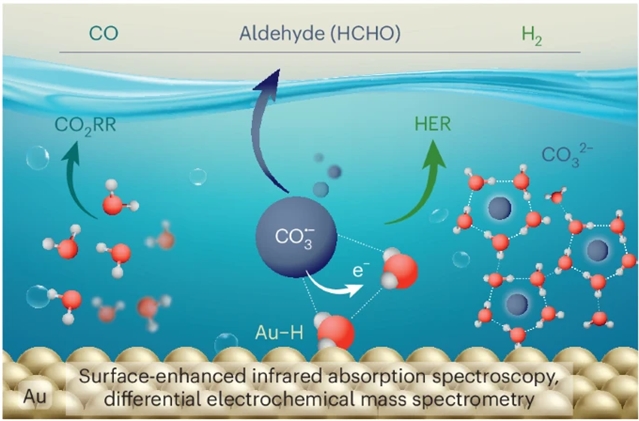
近日,德国马克斯·普朗克学会弗里茨·哈伯研究所Christopher S. Kley团队研究了碳酸盐阴离子和自由基诱导CO2电还原金的界面水有序。2025年11月25日出版的《自然-化学》杂志发表了这项成果。
界面水合层对能量和化学转换过程具有决定性作用,尤其影响电催化反应的动力学。由于难以原位解析水合结构与反应中间体及产物的关联,CO2电还原和析氢反应等过程的基本机制仍存在争议。
研究组通过振动和电化学光谱结合理论计算,揭示了碳酸盐如何排列界面水,从而调控金电催化剂在碳酸氢盐电解液中的CO2电还原和析氢反应。高阴极电位通过碳酸盐分子与其阴离子自由基平衡状态诱导的有序界面水合网络快速传递质子,加速了析氢反应;这些阴离子自由基除CO2外还可作为CO和醛类产物的碳源。此外,研究组证实水是CO2电还原和析氢反应的主要质子供体,碳酸氢盐主要参与海罗夫斯基步骤。这些分子层面的见解对电化学界面的理性设计和优化具有重要意义。
附:英文原文
Title: Carbonate anions and radicals induce interfacial water ordering in CO2 electroreduction on gold
Author: Zhou, Ya-Wei, Ibez-Al, Enric, Lpez, Nria, Roldan Cuenya, Beatriz, Kley, Christopher S.
Issue&Volume: 2025-11-25
Abstract: Interfacial hydration layers critically determine energy and chemical conversion processes, notably influencing the kinetics of electrocatalytic reactions. Fundamental mechanisms of reactions such as CO2 electroreduction and hydrogen evolution remain controversial due to the challenge of in situ deciphering of hydration structures alongside reaction intermediates and products. Here, by using vibrational and electrochemical spectroscopy paired with theory we reveal how carbonates structure interfacial water, affecting CO2 electroreduction and hydrogen evolution reactions on gold electrocatalysts in bicarbonate electrolytes. High cathodic potentials accelerate hydrogen evolution reactions by rapid proton delivery from ordered interfacial hydration networks, induced by carbonate molecules in equilibrium with their anion radicals. These radicals can serve, in addition to CO2, as a carbon source for CO and aldehyde production. Moreover we show water to be the primary proton donor for CO2 electroreduction and hydrogen evolution reactions, with bicarbonate mostly participating in the Heyrovsky step. Our molecular-level insights are relevant to rationalizing and optimizing electrochemical interfaces. The kinetics and product selectivity of electrocatalytic reactions depend on interfacial hydration, probing hydration structures alongside reaction intermediates and products is challenging. Now it has been shown that carbonates structure interfacial water during CO2 electroreduction and exist in equilibrium with their radicals, which serve various roles during the electrochemical processes.
DOI: 10.1038/s41557-025-01977-8
Source: https://www.nature.com/articles/s41557-025-01977-8
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
