德国慕尼黑大学院Roland Beckmann小组研究出碰撞核糖体的ZAK激活。2025年11月19日出版的《自然》发表了这项成果。
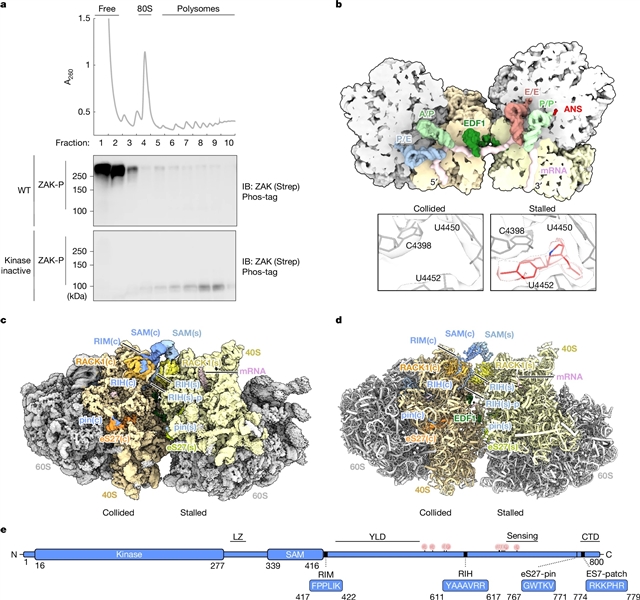
在这里,该研究组结合生物化学和低温电子显微镜来发现不同的ZAK-核糖体相互作用所需的组成招募和激活。该团队发现,在诱导核糖体碰撞时,ZAK与核糖体蛋白RACK1之间的相互作用使其在碰撞界面上通过其SAM结构域的二聚化而激活。
此外,该研究组发现该过程如何受到核糖体结合蛋白SERBP1的负调控,以阻止构成性ZAK激活。新的SAM变体以及已知的ZAK SAM结构域的致病变体的特征支持SAM结构域在调节核糖体内外激酶活性方面的关键作用,一些突变体绕过了ZAK激活所需的核糖体。总的来说,他们的数据提供了碰撞核糖体界面上ZAK激酶活性的机制蓝图。
据了解,核糖体碰撞激活由MAP3K - ZAK介导的核糖体毒性应激反应,进而通过MAPKs p38和JNK1的下游磷酸化调节细胞命运的后果。尽管ZAK在细胞应激过程中起着关键作用,但对ZAK -核糖体相互作用的机制和结构的理解以及这些相互作用如何导致激活仍然是未知的。
附:英文原文
Title: ZAK activation at the collided ribosome
Author: Huso, Vienna L., Niu, Shuangshuang, Catipovic, Marco A., Saba, James A., Denk, Timo, Park, Eugene, Cheng, Jingdong, Berninghausen, Otto, Becker, Thomas, Green, Rachel, Beckmann, Roland
Issue&Volume: 2025-11-19
Abstract: Ribosome collisions activate the ribotoxic stress response mediated by the MAP3K ZAK, which in turn regulates cell-fate consequences through downstream phosphorylation of the MAPKs p38 and JNK1. Despite the critical role of ZAK during cellular stress, a mechanistic and structural understanding of ZAK–ribosome interactions and how these lead to activation remain elusive. Here we combine biochemistry and cryo-electron microscopy to discover distinct ZAK–ribosome interactions required for constitutive recruitment and for activation. We find that upon induction of ribosome collisions, interactions between ZAK and the ribosomal protein RACK1 enable its activation by dimerization of its SAM domains at the collision interface. Furthermore, we discover how this process is negatively regulated by the ribosome-binding protein SERBP1 to prevent constitutive ZAK activation. Characterization of novel SAM variants as well as a known pathogenic variant of the SAM domain of ZAK supports a key role of the SAM domain in regulating kinase activity on and off the ribosome, with some mutants bypassing the ribosome requirement for ZAK activation. Collectively, our data provide a mechanistic blueprint of the kinase activity of ZAK at the collided ribosome interface.
DOI: 10.1038/s41586-025-09772-8
Source: https://www.nature.com/articles/s41586-025-09772-8
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
