芝加哥大学Robert J. Keenan课题组与斯坦福大学Rajat Rohatgi课题组宣布他们研制了分泌型翻译子调控N-糖基化的结构基础。该研究于2025年11月19日发表于国际一流学术期刊《自然》杂志上。
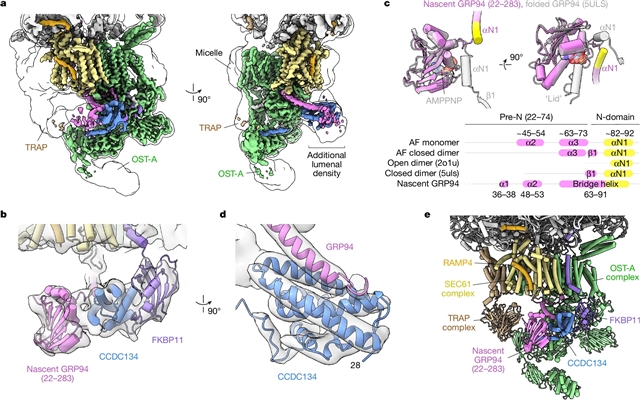
在这里,研究报道了一个天然分离的GRP94折叠中间体的结构,该结构连接到一个专门的CCDC134结合的转座子上。结合功能分析,这些数据揭示了GRP94中保守的N端延伸是如何抑制OST-A的,以及转座子内的结构重排是如何保护被束缚的新生链免受不适当的OST-B糖基化的。这些相互作用依赖于疏水的CCDC134沟槽,该沟槽识别新生GRP94的非天然构象。他们的研究结果定义了一种调节N-糖基化的机制,并阐明了新生链如何重塑转座子以促进其自身的生物发生。
研究人员表示,大多数人类分泌途径蛋白在进入内质网(ER)时被寡糖转移酶(OST)复合物N-糖基化。最近的研究揭示了一种底物辅助机制,通过调节伴侣糖调节蛋白94 (GRP94)的N-糖基化来控制细胞表面受体信号传导。
附:英文原文
Title: Structural basis of regulated N-glycosylation at the secretory translocon
Author: Yamsek, Melvin, Ma, Mengxiao, Jha, Roshan, Wan, Yu, Li, Qianru, Zhong, Frank, DeLong, Katherine, Ji, Zhe, Rohatgi, Rajat, Keenan, Robert J.
Issue&Volume: 2025-11-19
Abstract: Most human secretory pathway proteins are N-glycosylated by oligosaccharyltransferase (OST) complexes as they enter the endoplasmic reticulum (ER)1,2,3. Recent work revealed a substrate-assisted mechanism by which N-glycosylation of the chaperone glucose-regulated protein 94 (GRP94) is regulated to control cell surface receptor signalling4. Here we report the structure of a natively isolated GRP94 folding intermediate tethered to a specialized CCDC134-bound translocon. Together with functional analysis, the data reveal how a conserved N-terminal extension in GRP94 inhibits OST-A and how structural rearrangements within the translocon shield the tethered nascent chain from inappropriate OST-B glycosylation. These interactions depend on a hydrophobic CCDC134 groove, which recognizes a non-native conformation of nascent GRP94. Our results define a mechanism of regulated N-glycosylation and illustrate how the nascent chain remodels the translocon to facilitate its own biogenesis.
DOI: 10.1038/s41586-025-09756-8
Source: https://www.nature.com/articles/s41586-025-09756-8
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
