近日,美国密歇根大学Narayan, Alison R. H.团队研究了生物催化脱羧合成对映体富集阻转异构体。相关论文于2025年11月12日发表于《自然》杂志上。
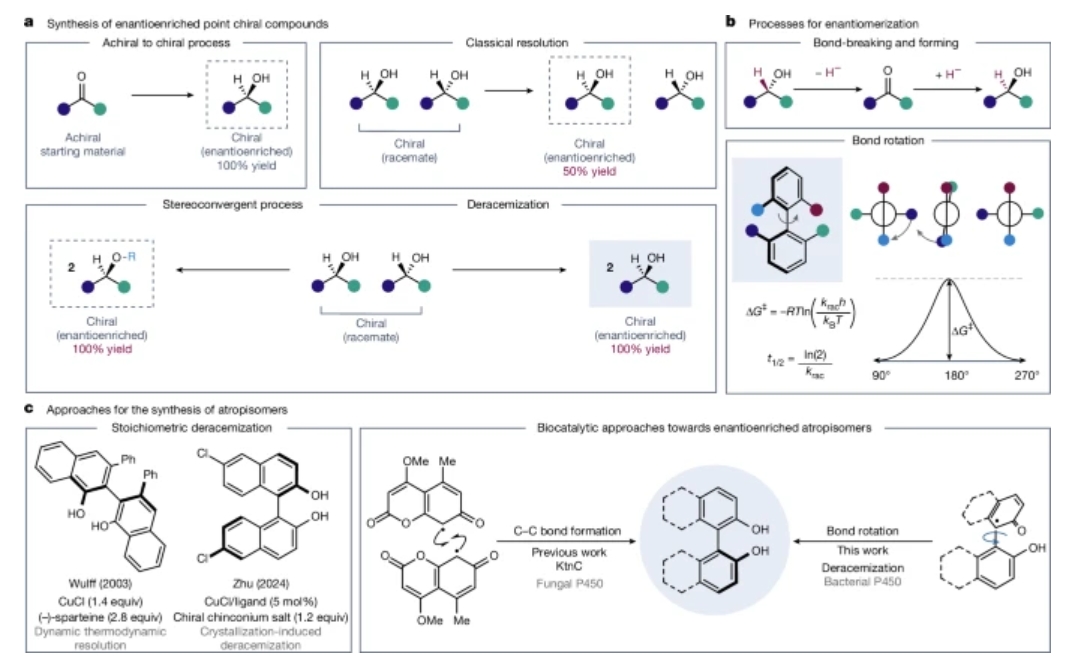
由于生物系统固有的手性特征,以及一对对映异构体可能具有显著不同生化性质的事实,对映纯材料的合成对于制药和农用化学品工业至关重要。其中,阻转异构体的对映选择性制备因其作为手性配体和药效团的独特地位而备受关注。虽然基于色谱或结晶的方法常用于分离阻转异构体,但人们迫切需要更高效、更经济的途径来获取高对映体富集的阻转异构体。利用立体汇聚方法获取具有点手性的分子已是成熟技术,但尚未发掘该方法在制备高对映体富集阻转异构体方面的潜力。
研究组报道了一种P450酶的去消旋化活性,并探索其实现高对映体富集阻转异构体的立体汇聚合成路径。通过筛选定制的P450变体库,研究组发现多种对称与非对称取代的2,2′-联萘酚结构单元均可通过去消旋化实现高对映体纯度。该去消旋化机制与既往报道的P450酶存在本质区别——后者通过对映选择性成键生成对映体富集阻转异构体,而该研究提出的去消旋化过程通过键旋转实现。由于工程化变体具有互补的选择性特征和底物范围,该生物催化平台可灵活适配各种取代模式。研究组预计这些研究成果将启发合成构象稳定阻转异构体的新型立体汇聚策略。
附:英文原文
Title: Synthesis of enantioenriched atropisomers by biocatalytic deracemization
Author: Roos, Casey B., Schulert, S. Luke, Zetzsche, Lara E., McMinn, Spencer E., Cheong, Angela E., Shim, Eunjae, Kwan, Eugene E., Narayan, Alison R. H.
Issue&Volume: 2025-11-12
Abstract: The synthesis of enantiopure materials is vital for pharmaceutical and agrochemical industries owing to the inherently chiral nature of biological systems and the fact that two enantiomers can have markedly different biochemical properties1. In particular, enantioselective preparation of atropisomers is of great interest owing to their privileged status as chiral ligands and pharmacophores2,3,4. Although chromatographic- or crystallization-based methods are commonly used to separate atropisomers, we urgently need more efficient and economical approaches to access enantioenriched atropisomers5,6. The use of stereoconvergent methods to access molecules with point chirality is well established but we have not tapped the potential of stereoconvergent catalytic methods to arrive at enantioenriched atropisomers. Here we report deracemization activity of a P450 enzyme and explore its ability to deliver a stereoconvergent route towards enantioenriched atropisomers. Using a curated set of P450 variants, we found that a wide variety of symmetric and non-symmetrically substituted 2,2′-binaphthol (BINOL) building blocks can be deracemized to high enantiomeric purity. This deracemization activity is mechanistically distinct from the activity of previously reported P450 enzymes, which operate through enantioselective bond formation to afford enantioenriched atropisomers. By contrast, the deracemization process reported here is proposed to proceed through bond rotation. As engineered variants have complementary selectivity profiles and substrate scope, this biocatalytic platform should be readily tunable for any desired substitution pattern. We anticipate that these results will inspire new stereoconvergent approaches to synthesizing conformationally stable atropisomers.
DOI: 10.1038/s41586-025-09738-w
Source: https://www.nature.com/articles/s41586-025-09738-w
官方网址:http://www.nature.com/
