2025年10月8日出版的《自然》杂志发表了美国梅奥诊所楼振昆小组的最新成果,研究探明了KCTD10是一个共向转录-复制冲突的传感器。
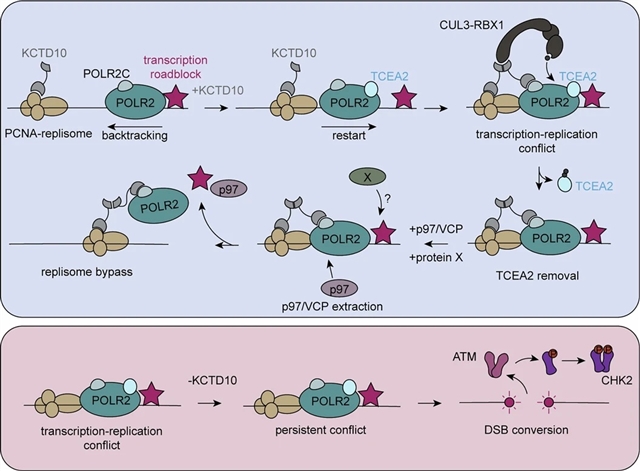
在这里,课题组发现CUL3-KCTD10 E3连接酶感知TRCs并促进RNA聚合酶复合物的重塑以允许复制体绕过。研究组发现底物接头KCTD10与复制体和转录机制相互作用,并在非胁迫条件下调节两者。这些二价相互作用允许KCTD10检测共向TRCs,并促进KCTD10复合物的高阶组装,这些复合物招募CUL3诱导泛素化和RNA聚合酶因子TCEA2的去除。在缺乏KCTD10的情况下,TCEA2和RNA聚合酶复合物的保留增加,导致TRCs的积累和DNA损伤增加。他们的研究结果表明,利用CUL3-KCTD10复合物的独特桥接功能,复制如何通过转录活跃区域进行。这些发现为转录和复制之间的协调如何有助于维持基因组稳定性提供了一个框架。
据介绍,在DNA复制过程中,复制体会移除包括转录机制在内的障碍和路障。当复制体和转录机制发生碰撞时,转录复制冲突(TRCs)就会发生,并且越来越被认为是哺乳动物基因组不稳定的一个重要原因。细胞如何促进TRCs位点的复制体旁路尚不完全清楚。
附:英文原文
Title: KCTD10 is a sensor for co-directional transcription–replication conflicts
Author: Kloeber, Jake A., Chen, Bin, Sun, Guangchao, King, Charles S., Wang, Zhiquan, Wang, Li, Wu, Zheming, Zhu, Shouhai, Zhao, Fei, Qin, Hongran, Ouyang, Yaobin, Xiao, Huaping, Tu, Xinyi, Lu, Jing, Jiang, Yanxia, Luo, Kuntian, Yin, Ping, Wu, Xinyan, Mutter, Robert W., Huang, Jinzhou, Lou, Zhenkun
Issue&Volume: 2025-10-08
Abstract: During DNA replication, the replisome must remove barriers and roadblocks including the transcription machinery1,2. Transcription–replication conflicts (TRCs) occur when there are collisions between the replisome and transcription machinery, and are increasingly recognized as an important source of mammalian genome instability3. How cells facilitate replisome bypass at sites of TRCs is incompletely understood. Here we show that the CUL3–KCTD10 E3 ligase senses TRCs and promotes remodelling of the RNA polymerase complex to allow replisome bypass. We found that the substrate adaptor KCTD10 interacts with the replisome and the transcription machinery and regulates both in unstressed conditions. These bivalent interactions allow KCTD10 to detect co-directional TRCs and facilitate higher-order assembly of KCTD10 complexes that recruit CUL3 to induce the ubiquitination and removal of the RNA polymerase factor TCEA2. In the absence of KCTD10, there is increased retention of TCEA2 and the RNA polymerase complex, causing an accumulation of TRCs and increased DNA damage. Our results demonstrate how replication can proceed through transcriptionally active regions, utilizing a unique bridging function of the CUL3–KCTD10 complex. These findings provide a framework for how the coordination between transcription and replication may contribute to the maintenance of genome stability.
DOI: 10.1038/s41586-025-09585-9
Source: https://www.nature.com/articles/s41586-025-09585-9
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
