近日,德国慕尼黑工业大学Hacker, Stephan M.团队分析了不同亲电试剂的蛋白质组选择性。这一研究成果于2025年10月30日发表在《自然-化学》杂志上。
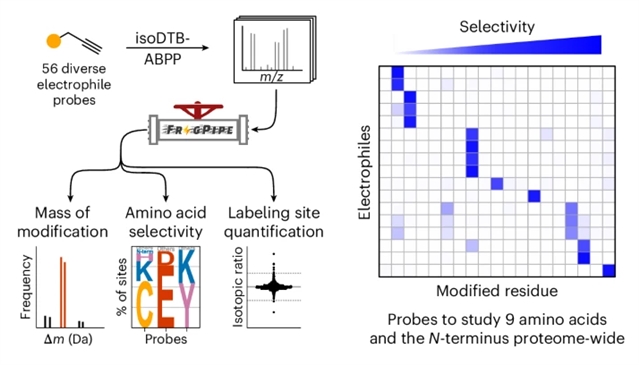
不依赖于劫持酶活性的共价抑制剂主要局限于那些针对半胱氨酸残基的抑制剂。这种半胱氨酸导向的共价抑制剂的开发很大程度上得益于竞争残基特异性蛋白质组学的应用,以确定其蛋白质组范围的选择性。目前已经开发了几种探针来监测其他氨基酸,并且存在更多的亲电试剂来修饰蛋白质。然而,对不同亲电试剂的选择性缺乏直接的、蛋白质组范围内的比较。
研究组开发了一种无偏倚的工作流程来分析亲电试剂在蛋白质组范围内的选择性,并将其用于直接比较含有不同反应基团的56个炔探针。通过这种方式,研究组验证并鉴定了探针,以监测整个蛋白质组中总共九种不同的氨基酸,以及它们的蛋白质氨基末端。
附:英文原文
Title: Profiling the proteome-wide selectivity of diverse electrophiles
Author: Zanon, Patrick R. A., Yu, Fengchao, Musacchio, Patricia Z., Lewald, Lisa, Zollo, Michael, Krauskopf, Kristina, Mrdovi, Dario, Raunft, Patrick, Maher, Thomas E., Cigler, Marko, Chang, Christopher J., Lang, Kathrin, Toste, F. Dean, Nesvizhskii, Alexey I., Hacker, Stephan M.
Issue&Volume: 2025-10-30
Abstract: Covalent inhibitors that do not rely on hijacking enzymatic activity have mainly been limited to those targeting cysteine residues. The development of such cysteine-directed covalent inhibitors has greatly profited from the use of competitive residue-specific proteomics to determine their proteome-wide selectivity. Several probes have been developed to monitor other amino acids using this technology, and many more electrophiles exist to modify proteins. Nevertheless, there has been a lack of direct, proteome-wide comparisons of the selectivity of diverse electrophiles. Here we developed an unbiased workflow to analyse electrophile selectivity proteome-wide and used it to directly compare 56 alkyne probes containing diverse reactive groups. In this way, we verified and identified probes to monitor a total of nine different amino acids, as well as the protein amino terminus, across the proteome.
DOI: 10.1038/s41557-025-01902-z
Source: https://www.nature.com/articles/s41557-025-01902-z
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
