近日,西班牙巴塞罗那科技学院Krieg, Michael团队揭示了障碍物调节膜张力传播实现局部机械传导。该研究于2025年10月29日发表在《自然—物理学》杂志上。
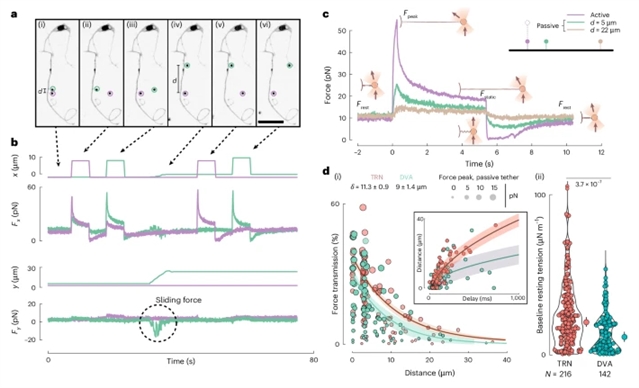
施加在细胞膜上的力导致瞬态膜张力梯度。膜张力从刺激位点向膜库传播的方式是细胞适应的一个关键特性。然而,目前尚不清楚张力在膜中的传播是如何调节的,以及它如何依赖于细胞类型。
研究组分析了培养秀丽隐杆线虫机械感觉神经元的质膜张力增殖。结果表明,张力传播速度很快,并且在神经元细胞体的神经突中被限制在特定的距离内。张力传播的生物物理模型表明,周期性障碍物的密度和排列在控制机械信息的传播中起着关键作用。他们的实验表明,张力传播强烈依赖于完整的肌动蛋白和微管细胞骨架,而膜脂性质的影响最小。
特别是,α/β-谱蛋白网络和MEC-2气孔蛋白凝聚物在周期性支架中的组织作为张力传播的屏障,限制了张力的传播。该发现表明,限制膜张力在空间和时间上的传播可以实现精确的局部信号,实现单个神经元在多个不同的领域处理机械信号,从而扩展其计算能力。
附:英文原文
Title: Obstacles regulate membrane tension propagation to enable localized mechanotransduction
Author: Catal-Castro, Frederic, Bonilla-Quintana, Mayte, Sanfeliu-Cerdn, Neus, Rangamani, Padmini, Krieg, Michael
Issue&Volume: 2025-10-29
Abstract: Forces applied to cellular membranes lead to transient membrane tension gradients. The way membrane tension propagates away from the stimulus site into the membrane reservoir is a key property in cellular adaptation. However, it remains unclear how tension propagation in membranes is regulated and how it depends on the cell type. Here we investigate plasma membrane tension propagation in cultured Caenorhabditis elegans mechanosensory neurons. We show that tension propagation travels quickly and is restricted to a particular distance in neurites—projections from the cell body of a neuron. A biophysical model of tension propagation suggests that periodic obstacle density and arrangement play key roles in controlling the propagation of mechanical information. Our experiments show that tension propagation is strongly dependent on the intact actin and microtubule cytoskeleton, whereas membrane lipid properties have a minimal impact. In particular, organization of the α/β-spectrin network and the MEC-2 stomatin condensates in a periodic scaffold acts as barriers to tension propagation, limiting the spread of tension. Our findings suggest that restricting membrane tension propagation in space and time enables precise localized signalling, allowing a single neuron to process mechanical signals in multiple distinct domains and, thus, expanding its computational capacity.
DOI: 10.1038/s41567-025-03037-x
Source: https://www.nature.com/articles/s41567-025-03037-x
