近日,南方科技大学刘心元团队报道了铜催化的不对称交叉偶联反应耐受高活性自由基。相关论文于2025年10月20日发表在《自然-化学》杂志上。
在不对称催化中实现高的对映体选择性,特别是对自由基等反应性很强的物质,往往是以牺牲通用性为代价的。具有极高反应活性的自由基通常不适合现有的不对称方法。
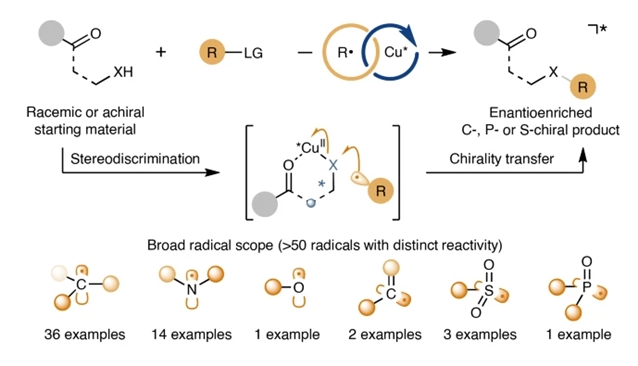
研究组提出了一种不对称自由基交叉偶联的通用催化方法,将铜催化的对映选择性立体中心分解或形成与铜介导的手性转移自由基取代相结合。这种顺序策略使50多种不同的碳、氮、氧、硫和磷中心自由基(包括高活性的甲基、叔丁基和苯基自由基)有效偶联,产生结构多样的C-、P-和S-手性化合物,具有出色的对映选择性。该方法为碳、磷、硫立体中心的合成提供了统一的平台,对药物化学及相关学科手性分子的制备具有重要意义。
此外,这种顺序立体识别和手性转移策略为开发适用于自由基以外的其他高活性物质的高对映选择性方法提供了一个有前景的蓝图。
附:英文原文
Title: Copper-catalysed asymmetric cross-coupling reactions tolerant of highly reactive radicals
Author: Fan, Li-Wen, Tang, Jun-Bin, Wang, Li-Lei, Gao, Zeng, Liu, Ji-Ren, Zhang, Yu-Shuai, Yuan, Dai-Lei, Qin, Li, Tian, Yu, Chen, Zhi-Chao, Liu, Fu, Xiang, Jin-Min, Huang, Pei-Jie, Liu, Wei-Long, Xiao, Chen-Yu, Luan, Cheng, Li, Zhong-Liang, Hong, Xin, Dong, Zhe, Gu, Qiang-Shuai, Liu, Xin-Yuan
Issue&Volume: 2025-10-20
Abstract: Achieving high enantioselectivity in asymmetric catalysis, especially with very reactive species such as radicals, often comes at the expense of generality. Radicals with exceptionally high reactivity are typically unsuitable for existing asymmetric methodologies. Here we present a general catalytic approach to asymmetric radical cross-coupling that combines copper-catalysed enantioselective stereocentre resolution or formation with copper-mediated, chirality-transferring radical substitution. This sequential strategy enables the efficient coupling of over 50 distinct carbon-, nitrogen-, oxygen-, sulfur- and phosphorus-centred radicals, including highly reactive methyl, tert-butoxyl and phenyl radicals, yielding structurally diverse C-, P- and S-chiral compounds with outstanding enantioselectivity. Our method thus provides a unified platform for the synthesis of carbon, phosphorus and sulfur stereocentres, with important implications for the preparation of chiral molecules relevant to medicinal chemistry and related disciplines. Furthermore, this sequential stereodiscrimination and chirality transfer strategy offers a promising blueprint for the development of highly enantioselective methodologies applicable to other classes of highly reactive species beyond radicals.
DOI: 10.1038/s41557-025-01970-1
Source: https://www.nature.com/articles/s41557-025-01970-1
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
