近日,英国曼彻斯特大学Fumagalli, L.团队研究了承压水的平面内介电常数和电导率。2025年10月15日出版的《自然》杂志发表了这项成果。
水对地球上几乎所有生命活动都至关重要,其性质已被深入探究,这并不令人意外。然而,人们对界面水和强限域水的电学性质仍知之甚少——这类水的结构不同于体相水,呈现出显著的分层特征。这种结构变化预计会影响水的导电性,尤其会改变其极化率,进而调控在众多物理化学过程中起关键作用的分子间作用力。
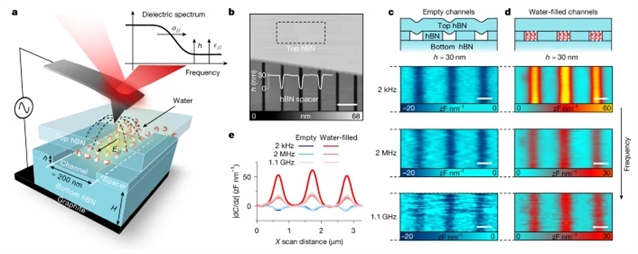
研究组采用扫描介电显微镜技术,探测了在间距小至1纳米的原子级平整表面间受限水的面内电学性质。当限域尺度超过数纳米时,水的面内介电常数接近体相水,其质子电导率显著增强,且随水膜厚度减小持续上升。但是当受限水仅剩数个分子层厚度时,该趋势发生突变:其面内介电常数达到约1000的极高值(呈现类铁电性),电导率则在几个S·m-1处达到峰值,接近超离子液体的典型数值。
研究组将这种增强效应归因于少数分子层限域诱导的强烈无序氢键网络,这种结构既促进了分子偶极子的面内极化,也加速了质子转移过程。对纳米限域水电学特性的这一认识,对于理解水界面和纳米孔道中发生的诸多现象具有重要意义。
附:英文原文
Title: In-plane dielectric constant and conductivity of confined water
Author: Wang, R., Souilamas, M., Esfandiar, A., Fabregas, R., Benaglia, S., Nevison-Andrews, H., Yang, Q., Normansell, J., Ares, P., Ferrari, G., Principi, A., Geim, A. K., Fumagalli, L.
Issue&Volume: 2025-10-15
Abstract: Water is essential for almost every aspect of life on our planet and, unsurprisingly, its properties have been studied in great detail1. However, disproportionately little remains known about the electrical properties of interfacial and strongly confined water2,3, in which the structure deviates from that of bulk water, becoming distinctly layered4,5. The structural change is expected to affect the conductivity of water and particularly its polarizability, which in turn modifies intermolecular forces that play a crucial role in many physical and chemical processes6,7,8,9. Here we use scanning dielectric microscopy (SDM)10 to probe the in-plane electrical properties of water confined between atomically flat surfaces separated by distances down to 1nm. For confinement exceeding several nanometres, water exhibits an in-plane dielectric constant close to that of bulk water and its proton conductivity is notably enhanced, gradually increasing with decreasing water thickness. This trend abruptly changes when the confined water becomes only a few molecules thick. Its in-plane dielectric constant reaches large, ferroelectric-like values of about 1,000, whereas the conductivity peaks at several Sm1, close to values characteristic of superionic liquids. We attribute the enhancement to strongly disordered hydrogen bonding induced by the few-layer confinement, which facilitates both easier in-plane polarization of molecular dipoles and faster proton exchange. This insight into the electrical properties of nanoconfined water is important for understanding many phenomena that occur at aqueous interfaces and in nanoscale pores.
DOI: 10.1038/s41586-025-09558-y
Source: https://www.nature.com/articles/s41586-025-09558-y
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
