澳大利亚莫纳什大学Jeremy J. Barr小组宣布他们研制了人类肠道温和噬菌体的分离、改造与生态学研究。2025年10月15日出版的《自然》发表了这项成果。
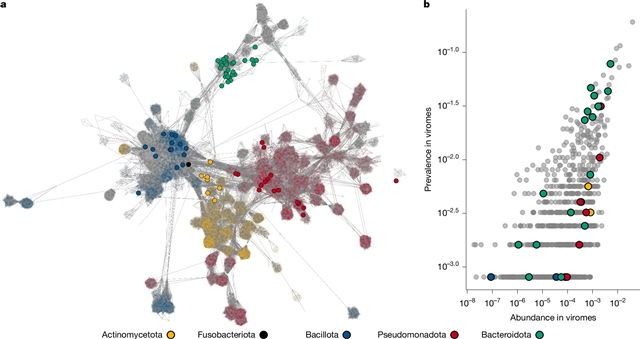
在这里,小组对来自252个人类肠道细菌分离株的134个可诱导的噬菌体进行了表征,通过10种不同的诱导条件来扩增实验验证的来自人类肠道的温带噬菌体-宿主对。重要的是,只有18%的计算预测的噬菌体可以在纯培养物中诱导。
此外,研究人员构建了一个由78个成员组成的合成微生物组,当与人类结肠细胞(Caco2)共培养时,诱导了35%的噬菌体物种。通过培养的分离物,研究团队证明了人类宿主相关细胞产物可能作为诱导剂,在胃肠道细胞裂解和温带噬菌体种群之间提供了可能的联系。研究团队提供了关于前噬菌体多样性和遗传学的关键见解,包括驯化的遗传途径,发现多溶生是常见的,导致前噬菌体的协调诱导,并且差异诱导可能受到不同的前噬菌体整合位点的影响。更广泛地说,他们的研究强调了基于培养的技术,以及实验验证,基因组学和计算预测的重要性,以了解人类肠道微生物组中温带噬菌体的生物学和功能。这些基于培养的方法将使合成生物学、生物技术和微生物学领域的应用成为可能。
据介绍,大规模的宏基因组和数据挖掘工作已经揭示了人类肠道内噬菌体(噬菌体)的广泛多样性。然而,在这种复杂的环境中,对噬菌体-宿主相互作用的功能理解是有限的,主要是由于缺乏可用于实验验证的培养分离物。
附:英文原文
Title: Isolation, engineering and ecology of temperate phages from the human gut
Author: Dahlman, Sofia, Avellaneda-Franco, Laura, Rutten, Emily L., Gulliver, Emily L., Solari, Sean, Chonwerawong, Michelle, Kett, Ciaren, Subedi, Dinesh, Young, Remy B., Campbell, Nathan, Gould, Jodee A., Bell, Jasmine D., Docherty, Callum A. H., Turkington, Christopher J. R., Nezam-Abadi, Neda, Grasis, Juris A., Lyras, Dena, Edwards, Robert A., Forster, Samuel C., Barr, Jeremy J.
Issue&Volume: 2025-10-15
Abstract: Large-scale metagenomic and data-mining efforts have revealed an expansive diversity of bacteriophages (phages) within the human gut1,2,3. However, functional understanding of phage–host interactions within this complex environment is limited, largely due to a lack of cultured isolates available for experimental validation. Here we characterize 134 inducible prophages originating from 252 human gut bacterial isolates using 10 different induction conditions to expand the experimentally validated temperate phage–host pairs originating from the human gut. Importantly, only 18% of computationally predicted prophages could be induced in pure cultures. Moreover, we construct a 78-member synthetic microbiome that, when co-cultured in the presence of human colonic cells (Caco2), led to the induction of 35% phage species. Using cultured isolates, we demonstrate that human host-associated cellular products may act as induction agents, providing a possible link between gastrointestinal cell lysis and temperate phage populations4,5. We provide key insights into prophage diversity and genetics, including a genetic pathway for domestication, finding that polylysogeny was common and resulted in coordinated prophage induction, and that differential induction can be influenced by divergent prophage integration sites. More broadly, our study highlights the importance of culture-based techniques, alongside experimental validation, genomics and computational prediction, to understand the biology and function of temperate phages in the human gut microbiome. These culture-based approaches will enable applications across synthetic biology, biotechnology and microbiome fields.
DOI: 10.1038/s41586-025-09614-7
Source: https://www.nature.com/articles/s41586-025-09614-7
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
