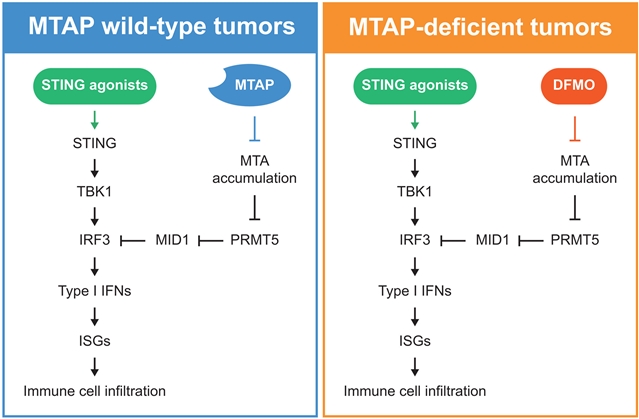
该课题组人员发现,在人类肿瘤中,纯合子MTAP的缺失通过下调干扰素调节因子3 (IRF3),形成一个肿瘤微环境,阻碍细胞质核酸感应途径,导致对STING激动剂产生耐药性。靶向多胺生物合成逆转IRF3下调,恢复MTAP缺陷肿瘤对STING激动剂的敏感性。他们的研究结果表明,MTAP基因状态可能会影响患者对STING激动剂治疗的反应,并为在MTAP缺失的肿瘤中增强以STING激动剂为主体的抗肿瘤免疫反应提供了一种替代策略。
据了解,细胞质核酸感应途径是癌症免疫治疗的潜在靶点。尽管干扰素基因刺激剂(STING)激动剂在动物模型中显示出显著的抗肿瘤作用,但其在人类肿瘤中的临床疗效尚不清楚。甲基硫代腺苷磷酸化酶(MTAP)的缺失是人类肿瘤中常见的基因组改变,但在临床前同基因基序模型中很少见。
附:英文原文
Title: MTAP deficiency confers resistance to cytosolic nucleic acid sensing and STING agonists
Author: Jung-Mao Hsu, Chunxiao Liu, Weiya Xia, Chung-Yu Chen, Wei-Chung Cheng, Junwei Hou, Mien-Chie Hung
Issue&Volume: 2025-10-09
Abstract: Cytosolic nucleic acid–sensing pathways are potential targets for cancer immunotherapy. Although stimulator of interferon genes (STING) agonists have shown substantial antitumor effects in animal models, their clinical efficacy in human tumors remains unclear. Deletion of methylthioadenosine phosphorylase (MTAP) is a common genomic alteration in human tumors but is rare in preclinical syngeneic mouse models. We found that homozygous MTAP deletion in human tumors creates a tumor microenvironment that obstructs cytosolic nucleic acid–sensing pathways by down-regulating interferon regulatory factor 3 (IRF3), leading to resistance to STING agonists. Targeting polyamine biosynthesis reverses IRF3 down-regulation, restoring sensitivity to STING agonists in MTAP-deficient tumors. Our findings suggest that MTAP genetic status may inform patient responses to STING agonist therapy and offer an alternative strategy for boosting antitumor immune responses using STING agonists in MTAP-deleted tumors.
DOI: adl4089
Source: https://www.science.org/doi/10.1126/science.adl4089
