美国圣裘德儿童研究医院Chia-Hsueh Lee等研究人员合作揭示对Spns2介导的鞘氨醇-1-磷酸运输的结构和功能的见解。相关论文于2023年5月23日在线发表在《细胞》杂志上。
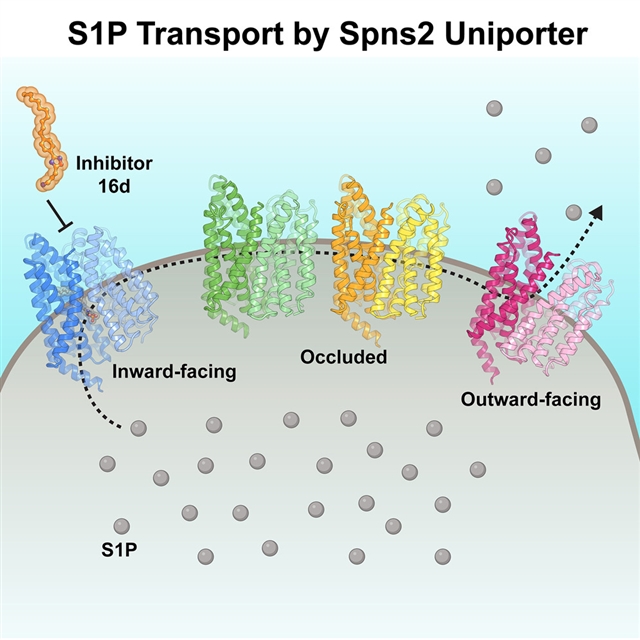
研究人员展示了人类Spinster homolog 2(Spns2)在脂质纳米盘中的六个冷冻电镜结构,包括两个与功能有关的中间构象,连接内向和外向状态,从而揭示出鞘氨醇-1-磷酸(S1P)运输周期的结构基础。功能分析表明,Spns2通过促进扩散输出S1P,这种机制与其他MFS脂质运输工具不同。最后,研究人员显示,Spns2抑制剂16d将Spns2锁定在向内的状态,从而减弱了其运输活动。这项工作揭示了Spns2介导的S1P运输,有助于开发先进的Spns2抑制剂。
据介绍,S1P是一种重要的信号鞘脂,可调节免疫系统、血管生成、听觉功能以及上皮细胞和内皮细胞屏障的完整性。Spns2是一种S1P转运器,输出S1P来启动脂质信号级联。调节Spns2的活性对治疗癌症、炎症和免疫性疾病有好处。然而,Spns2的运输机制和它的抑制作用仍然不清楚。
附:英文原文
Title: Structural and functional insights into Spns2-mediated transport of sphingosine-1-phosphate
Author: Hongwen Chen, Shahbaz Ahmed, Hongtu Zhao, Nadia Elghobashi-Meinhardt, Yaxin Dai, Jae Hun Kim, Jeffrey G. McDonald, Xiaochun Li, Chia-Hsueh Lee
Issue&Volume: 2023-05-23
Abstract: Sphingosine-1-phosphate (S1P) is an important signaling sphingolipid that regulatesthe immune system, angiogenesis, auditory function, and epithelial and endothelialbarrier integrity. Spinster homolog 2 (Spns2) is an S1P transporter that exports S1Pto initiate lipid signaling cascades. Modulating Spns2 activity can be beneficialin treatments of cancer, inflammation, and immune diseases. However, the transportmechanism of Spns2 and its inhibition remain unclear. Here, we present six cryo-EMstructures of human Spns2 in lipid nanodiscs, including two functionally relevantintermediate conformations that link the inward- and outward-facing states, to revealthe structural basis of the S1P transport cycle. Functional analyses suggest thatSpns2 exports S1P via facilitated diffusion, a mechanism distinct from other MFS lipidtransporters. Finally, we show that the Spns2 inhibitor 16d attenuates the transportactivity by locking Spns2 in the inward-facing state. Our work sheds light on Spns2-mediatedS1P transport and aids the development of advanced Spns2 inhibitors.
DOI: 10.1016/j.cell.2023.04.028
Source: https://www.cell.com/cell/fulltext/S0092-8674(23)00457-9
