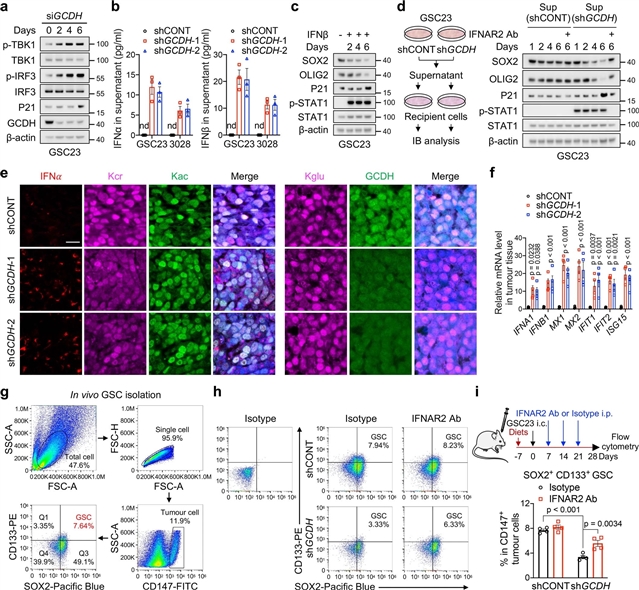
美国匹兹堡大学Jeremy N. Rich团队发现,赖氨酸分解代谢通过组蛋白巴豆酰化对肿瘤免疫进行重编程。2023年5月17日,《自然》杂志在线发表了这项成果。
研究人员发现,胶质母细胞瘤干细胞(GSC)通过上调赖氨酸转运体SLC7A2以及产生巴豆酰辅酶A(crotonyl-CoA)的谷氨酰辅酶(GCDH),下调巴豆酰辅酶水解酶enoyl-CoA水解酶短链1(ECHS1)来重新规划赖氨酸的分解代谢、 导致细胞内巴豆酰-CoA的积累和组蛋白H4赖氨酸的巴豆酰化。通过遗传操作或赖氨酸限制减少组蛋白赖氨酸巴豆酰化,会损害肿瘤的生长。在细胞核中,GCDH与巴豆酰转移酶CBP相互作用,促进组蛋白赖氨酸的巴豆酰化。组蛋白赖氨酸巴豆酰化的丧失通过增强H3K27ac促进免疫原性细胞双链RNA(dsRNA)和dsDNA的生成,从而刺激RNA传感器MDA5和DNA传感器环状GMP-AMP合成酶(cGAS)促进I型干扰素信号,导致GSC致瘤潜力受损和CD8+T细胞浸润升高。限制赖氨酸的饮食与MYC抑制或抗PD-1治疗协同作用,减缓肿瘤的生长。
总的来说,GSC共同选择赖氨酸的吸收和降解来分流巴豆酰-CoA的产生,并重塑染色质景观来逃避干扰素诱导的GSC维持的内在影响和免疫反应的外在影响。
据介绍,癌细胞重新调整代谢,以利于产生支持肿瘤生长和重塑肿瘤微环境的特殊代谢物。赖氨酸作为一种生物合成分子、能量来源和抗氧化剂,但对其在癌症中的病理作用知之甚少。
附:英文原文
Title: Lysine catabolism reprograms tumour immunity through histone crotonylation
Author: Yuan, Huairui, Wu, Xujia, Wu, Qiulian, Chatoff, Adam, Megill, Emily, Gao, Jinjun, Huang, Tengfei, Duan, Tingting, Yang, Kailin, Jin, Chunyu, Yuan, Fanen, Wang, Shuai, Zhao, Linjie, Zinn, Pascal O., Abdullah, Kalil G., Zhao, Yingming, Snyder, Nathaniel W., Rich, Jeremy N.
Issue&Volume: 2023-05-17
Abstract: Cancer cells rewire metabolism to favour the generation of specialized metabolites that support tumour growth and reshape the tumour microenvironment1,2. Lysine functions as a biosynthetic molecule, energy source and antioxidant3,4,5, but little is known about its pathological role in cancer. Here we show that glioblastoma stem cells (GSCs) reprogram lysine catabolism through the upregulation of lysine transporter SLC7A2 and crotonyl-coenzyme A (crotonyl-CoA)-producing enzyme glutaryl-CoA dehydrogenase (GCDH) with downregulation of the crotonyl-CoA hydratase enoyl-CoA hydratase short chain 1 (ECHS1), leading to accumulation of intracellular crotonyl-CoA and histone H4 lysine crotonylation. A reduction in histone lysine crotonylation by either genetic manipulation or lysine restriction impaired tumour growth. In the nucleus, GCDH interacts with the crotonyltransferase CBP to promote histone lysine crotonylation. Loss of histone lysine crotonylation promotes immunogenic cytosolic double-stranded RNA (dsRNA) and dsDNA generation through enhanced H3K27ac, which stimulates the RNA sensor MDA5 and DNA sensor cyclic GMP–AMP synthase (cGAS) to boost type I interferon signalling, leading to compromised GSC tumorigenic potential and elevated CD8+ T cell infiltration. A lysine-restricted diet synergized with MYC inhibition or anti-PD-1 therapy to slow tumour growth. Collectively, GSCs co-opt lysine uptake and degradation to shunt the production of crotonyl-CoA, remodelling the chromatin landscape to evade interferon-induced intrinsic effects on GSC maintenance and extrinsic effects on immune response.
DOI: 10.1038/s41586-023-06061-0
Source: https://www.nature.com/articles/s41586-023-06061-0
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
