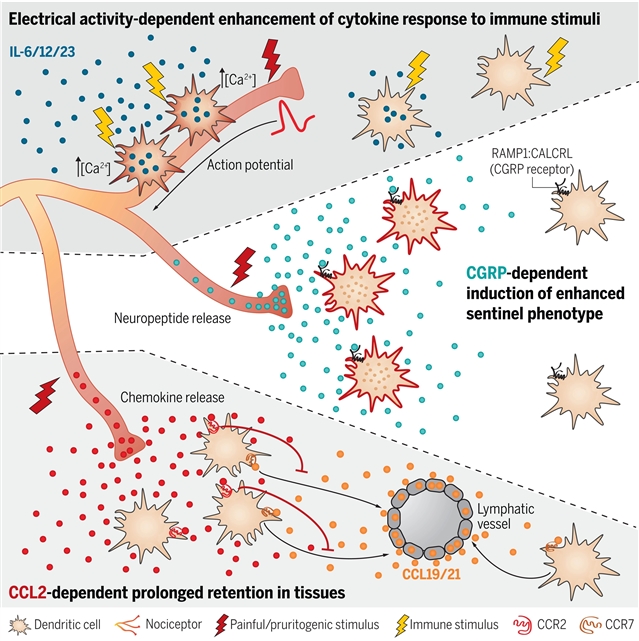
研究人员表明伤害感受器以三种不同的分子方式控制树突状细胞(DC)。首先,伤害感受器释放降钙素基因相关肽,在稳态DC上赋予独特的转录特征,即表达白细胞介素-1β和其他涉及DC哨兵功能的基因。其次,伤害感受器的激活诱导了DC中接触依赖性的钙通量和膜去极化,并在受到刺激时增强了它们对促炎症细胞因子的产生。最后,伤害感受器衍生的趋化因子CCL2有助于协调DC依赖性的局部炎症和诱导针对皮肤获得性抗原的适应性反应。因此,伤害感受器衍生的趋化因子、神经肽和电活动的联合作用,对屏障组织中的DC反应进行了微调。
据悉,众所周知,伤害感受器和DC之间的相互作用可以调节屏障组织的免疫反应。然而,人们对潜在的通信框架的理解仍然很不成熟。
附:英文原文
Title: Multimodal control of dendritic cell functions by nociceptors
Author: Pavel Han, Rodrigo J. Gonzalez, Irina B. Mazo, Yidi Wang, Talley Lambert, Gloria Ortiz, Evan W. Miller, Ulrich H. von Andrian
Issue&Volume: 2023-03-31
Abstract: It is known that interactions between nociceptors and dendritic cells (DCs) can modulate immune responses in barrier tissues. However, our understanding of the underlying communication frameworks remains rudimentary. Here, we show that nociceptors control DCs in three molecularly distinct ways. First, nociceptors release the calcitonin gene–related peptide that imparts a distinct transcriptional profile on steady-state DCs characterized by expression of pro–interleukin-1β and other genes implicated in DC sentinel functions. Second, nociceptor activation induces contact-dependent calcium fluxes and membrane depolarization in DCs and enhances their production of proinflammatory cytokines when stimulated. Finally, nociceptor-derived chemokine CCL2 contributes to the orchestration of DC-dependent local inflammation and the induction of adaptive responses against skin-acquired antigens. Thus, the combined actions of nociceptor-derived chemokines, neuropeptides, and electrical activity fine-tune DC responses in barrier tissues.
DOI: abm5658
Source: https://www.science.org/doi/10.1126/science.abm5658
