美国佛罗里达州立大学Celis-Barros, Cristian团队报道了锎(II)冠醚复合物的分离。相关研究成果发表在2023年3月27日出版的《自然—化学》。
锕系元素,从锎到铷(Z=98-102),已知具有可获得的+2氧化态。了解这种化学行为的起源需要对CfII材料进行表征,但由于它们仍然难以分离,研究受到阻碍。这在一定程度上是由于操纵这种不稳定元素的内在挑战,以及缺乏不将CfIII还原为Cf°的合适还原剂。
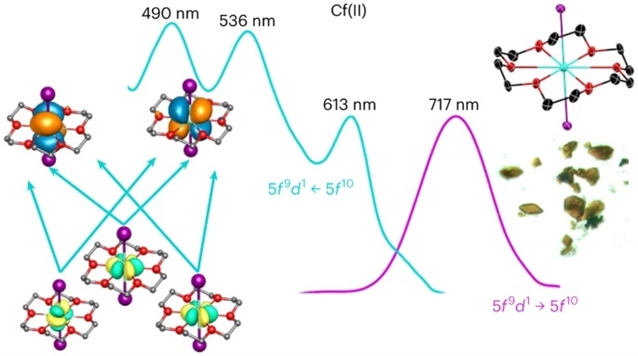
该文中,研究表明,使用Al/Hg汞合金作为还原剂,可以制备CfII冠醚络合物Cf(18-冠-6)I2。光谱证据表明,CfIII可以定量还原为CfII,并且在溶液中的快速辐解再氧化产生CfII和CfIII复合物的共结晶混合物,而不需要Al/Hg混合物。量子化学计算表明,Cf-配体的相互作用是高度离子性的,并且不存在5f/6d混合,从而导致弱5f→5f跃迁和5f→6d转换主导的吸收光谱。
附:英文原文
Title: Isolation of a californium(II) crown–ether complex
Author: Poe, Todd N., Ramanantoanina, Harry, Sperling, Joseph M., Wineinger, Hannah B., Rotermund, Brian M., Brannon, Jacob, Bai, Zhuanling, Scheibe, Benjamin, Beck, Nicholas, Long, Brian N., Justiniano, Samantha, Albrecht-Schnzart, Thomas E., Celis-Barros, Cristian
Issue&Volume: 2023-03-27
Abstract: The actinides, from californium to nobelium (Z=98–102), are known to have an accessible +2 oxidation state. Understanding the origin of this chemical behaviour requires characterizing CfII materials, but investigations are hampered by the fact that they have remained difficult to isolate. This partly arises from the intrinsic challenges of manipulating this unstable element, as well as a lack of suitable reductants that do not reduce CfIII to Cf°. Here we show that a CfII crown–ether complex, Cf(18-crown-6)I2, can be prepared using an Al/Hg amalgam as a reductant. Spectroscopic evidence shows that CfIII can be quantitatively reduced to CfII, and rapid radiolytic re-oxidation in solution yields co-crystallized mixtures of CfII and CfIII complexes without the Al/Hg amalgam. Quantum-chemical calculations show that the Cfligand interactions are highly ionic and that 5f/6d mixing is absent, resulting in weak 5f→5f transitions and an absorption spectrum dominated by 5f→6d transitions.
DOI: 10.1038/s41557-023-01170-9
Source: https://www.nature.com/articles/s41557-023-01170-9
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
