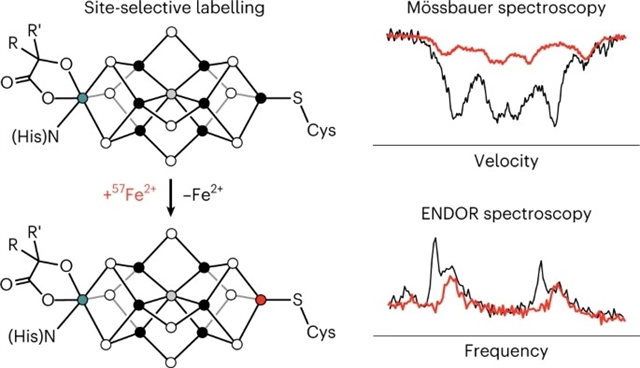
美国麻省理工学院Daniel L. M. Suess课题组通过位点选择性57Fe标记,成功将固氮酶铁–钼辅因子的几何结构和电子结构联系在一起。相关论文于2023年3月13日发表于国际顶尖学术期刊《自然—化学》杂志上。
了解钼固氮酶催化辅因子(FeMo-co)中的化学键是构建生物固氮机理的基础。实现这一目标的一个持久的障碍是基于57Fe的光谱数据,尽管其中信息丰富,但因为结合了所有7个Fe位点的响应,之前不可能将单个光谱响应对应到三维结构中的特定位点。
在该研究中,课题组人员通过将57Fe合并到FeMo-co的单个位点来解决这一挑战。静息态的光谱分析提供了末端Fe1位点的局部电子结构,包括其氧化态和自旋取向,以及整个团簇的自旋-耦合方式。氧化后静息态和固氮反应的第一个中间体也被研究者表征,其与静息态的比较提供了对FeMo-co氧化还原化学的分子水平的见解。
附:英文原文
Title: Connecting the geometric and electronic structures of the nitrogenase iron–molybdenum cofactor through site-selective 57Fe labelling
Author: Badding, Edward D., Srisantitham, Suppachai, Lukoyanov, Dmitriy A., Hoffman, Brian M., Suess, Daniel L. M.
Issue&Volume: 2023-03-13
Abstract: Understanding the chemical bonding in the catalytic cofactor of the Mo nitrogenase (FeMo-co) is foundational for building a mechanistic picture of biological nitrogen fixation. A persistent obstacle towards this goal has been that the 57Fe-based spectroscopic data—although rich with information—combines responses from all seven Fe sites, and it has therefore not been possible to map individual spectroscopic responses to specific sites in the three-dimensional structure. Here we have addressed this challenge by incorporating 57Fe into a single site of FeMo-co. Spectroscopic analysis of the resting state informed on the local electronic structure of the terminal Fe1 site, including its oxidation state and spin orientation, and, in turn, on the spin-coupling scheme for the entire cluster. The oxidized resting state and the first intermediate in nitrogen fixation were also characterized, and comparisons with the resting state provided molecular-level insights into the redox chemistry of FeMo-co.
DOI: 10.1038/s41557-023-01154-9
Source: https://www.nature.com/articles/s41557-023-01154-9
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
