美国哈佛医学院Philip J. Kranzusch研究团队揭示RADAR超分子抗噬菌体防御复合物的冷冻电镜结构。相关论文于2023年2月9日在线发表在《细胞》杂志上。
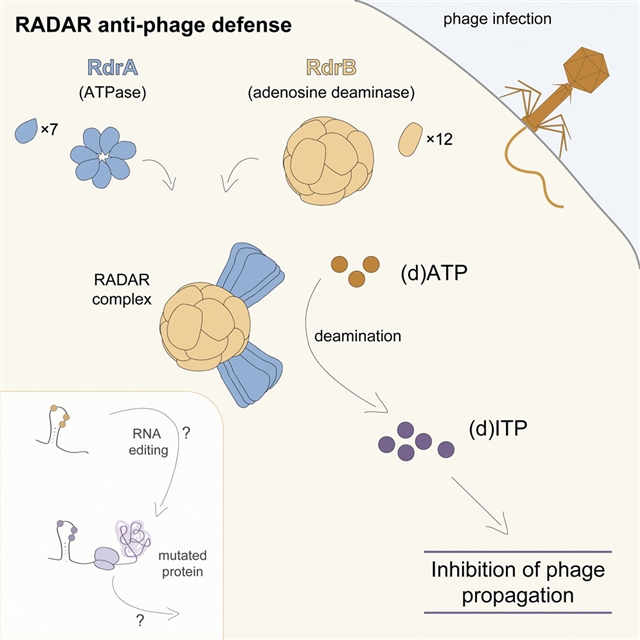
研究人员确定了RADAR防御复合物的冷冻电镜结构,并揭示RdrA为七聚体,两层AAA+ ATP酶,RdrB为十二聚体,空心复合物,有12个表面暴露的脱氨酶活性位点。RdrA和RdrB连接形成一个高达10个MDa的大型组件,RdrA作为一个漏斗停靠在RdrB活动站点之上。令人惊讶的是,这些结构揭示了一个针对单个核苷酸的RdrB活性位点。研究人员发现,RdrB在体外催化ATP到ITP的转化,并诱导体内噬菌体感染期间肌苷单核苷酸的大量积累,从而限制噬菌体复制。这些结果将ATP单核苷酸脱氨定义为RADAR免疫的决定因素,并揭示了核苷酸修饰机器的超分子组装作为抗噬菌体防御的机制。
据介绍,RADAR是一种双蛋白质细菌防御系统,据报道可以通过“编辑”信使RNA来防御噬菌体。
附:英文原文
Title: Cryo-EM structure of the RADAR supramolecular anti-phage defense complex
Author: Brianna Duncan-Lowey, Nitzan Tal, Alex G. Johnson, Shaun Rawson, Megan L. Mayer, Shany Doron, Adi Millman, Sarah Melamed, Taya Fedorenko, Assaf Kacen, Alexander Brandis, Tevie Mehlman, Gil Amitai, Rotem Sorek, Philip J. Kranzusch
Issue&Volume: 2023-02-09
Abstract: RADAR is a two-protein bacterial defense system that was reported to defend against phage by “editing” messenger RNA. Here, we determine cryo-EM structures of the RADAR defense complex, revealing RdrA as a heptameric, two-layered AAA+ ATPase and RdrB as a dodecameric, hollow complex with twelve surface-exposed deaminase active sites. RdrA and RdrB join to form a giant assembly up to 10 MDa, with RdrA docked as a funnel over the RdrB active site. Surprisingly, our structures reveal an RdrB active site that targets mononucleotides. We show that RdrB catalyzes ATP-to-ITP conversion in vitro and induces the massive accumulation of inosine mononucleotides during phage infection in vivo, limiting phage replication. Our results define ATP mononucleotide deamination as a determinant of RADAR immunity and reveal supramolecular assembly of a nucleotide-modifying machine as a mechanism of anti-phage defense.
DOI: 10.1016/j.cell.2023.01.012
Source: https://www.cell.com/cell/fulltext/S0092-8674(23)00042-9
