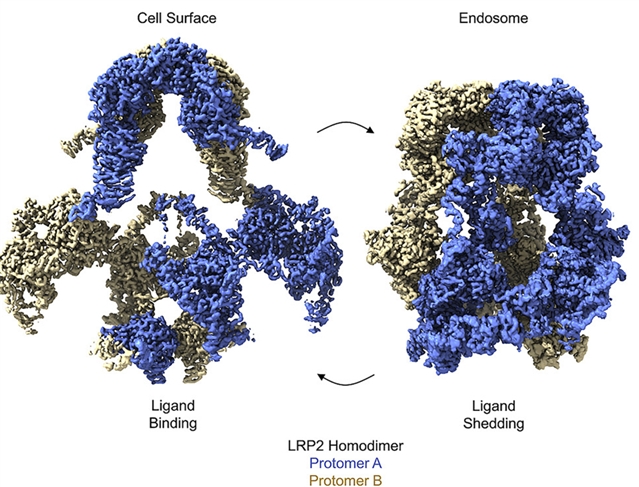
美国哥伦比亚大学Lawrence Shapiro等研究人员合作发现,LRP2的结构揭示了内吞作用的分子机器。这一研究成果于2023年2月6日在线发表在国际学术期刊《细胞》上。
研究人员报道了从小鼠肾脏中分离出的低密度脂蛋白(LDL)受体相关蛋白2(LRP2或megalin)高分辨率冷冻电镜结构,这些蛋白处于在细胞外的和内体的pH值中。这些结构揭示了LRP2是一种分子机器,它采用一种构象在细胞表面结合配体和在核内体中脱落配体。LRP2形成同源二聚体,其构象转化由同源二聚体和原聚体界面上的pH敏感位点控制。人类中,LRP2一个亚群的有害错义变体似乎损害了同源二聚体的组装。这些观察结果为进一步理解LDL受体的功能和机制奠定了基础,并暗示同型二聚化是LRP受体亚家族的保守特征。
据介绍,LRP2是系统发育保守的巨型LDL受体相关蛋白亚家族代表,该亚家族在内吞作用中起作用,并与肾脏和大脑疾病有关。
附:英文原文
Title: Structures of LRP2 reveal a molecular machine for endocytosis
Author: Andrew Beenken, Gabriele Cerutti, Julia Brasch, Yicheng Guo, Zizhang Sheng, Hediye Erdjument-Bromage, Zainab Aziz, Shelief Y. Robbins-Juarez, Estefania Y. Chavez, Goran Ahlsen, Phinikoula S. Katsamba, Thomas A. Neubert, Anthony W.P. Fitzpatrick, Jonathan Barasch, Lawrence Shapiro
Issue&Volume: 2023-02-06
Abstract: The low-density lipoprotein (LDL) receptor-related protein 2 (LRP2 or megalin) isrepresentative of the phylogenetically conserved subfamily of giant LDL receptor-relatedproteins, which function in endocytosis and are implicated in diseases of the kidneyand brain. Here, we report high-resolution cryoelectron microscopy structures of LRP2isolated from mouse kidney, at extracellular and endosomal pH. The structures revealLRP2 to be a molecular machine that adopts a conformation for ligand binding at thecell surface and for ligand shedding in the endosome. LRP2 forms a homodimer, theconformational transformation of which is governed by pH-sensitive sites at both homodimerand intra-protomer interfaces. A subset of LRP2 deleterious missense variants in humansappears to impair homodimer assembly. These observations lay the foundation for furtherunderstanding the function and mechanism of LDL receptors and implicate homodimerizationas a conserved feature of the LRP receptor subfamily.
DOI: 10.1016/j.cell.2023.01.016
Source: https://www.cell.com/cell/fulltext/S0092-8674(23)00046-6
